Research ArticleOpen Access, Volume 2 Issue 3
Bioethical Considerations in Leading Pharmaceutical Companies: A Comparative Analysis in BRICS Nations
Talib Hussain1,2,3*
1Department of Media and Communication, Shanghai Jiao Tong University, China.
2Faculty of Social Sciences, Media and Communication, University of Religions and Denominations, Qom, Iran.
3Karakoram International University, Gilgit-Baltistan, Pakistan.
*Corresponding author: Talib Hussain
Department of Media and Communication, Shanghai Jiao Tong University, China.
Email: talibhussian@sjtu.edu.cn
Received : Apr 16, 2024 Accepted : May 20, 2024 Published : May 27, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Hussain T © All rights are reserved
Citation: Hussain T. Bioethical Considerations in Leading Pharmaceutical Companies: A Comparative Analysis in BRICS Nations. Epidemiol Public Health. 2024; 2(3): 1053.
Abstract
This scholarly investigation offers a meticulous examination of bioethical considerations within the vision and mission statements of preeminent pharmaceutical corporations operating in the BRICS nations. Comprising Brazil, Russia, India, China, and South Africa, these emerging economic powerhouses possess pivotal pharmaceutical sectors of global consequence. The research method was painstakingly devised to scrutinize the ethical landscape within these nations, incorporating thematic analysis and an assessment grounded in the bioethical tenets articulated by the World Health Organization (WHO). The findings illuminate an array of bioethical commitments among BRICS nations, revealing a noteworthy divergence in the priority accorded to these considerations. Brazil, marked by the highest mean value, evinces a substantial emphasis on bioethical principles. However, the considerable standard deviation denotes a broad spectrum of approaches within Brazilian pharmaceutical companies, warranting further scrutiny. Russian pharmaceutical entities, with a moderately lower mean value, display a degree of commitment to bioethical considerations. The moderate standard deviation hints at nuances in the application of these principles, meriting exploration to elucidate specific contextual influences. Conversely, Indian pharmaceutical companies manifest the lowest mean value, suggesting a relatively lower emphasis on bioethical tenets. Although the standard deviation implies variability in bioethical commitments, the scope of these variations warrants meticulous examination. Chinese pharmaceutical companies, while ranking higher than India, also emphasize bioethics to a lesser degree. Notably, the standard deviation mirrors the mean, signifying uniformity in bioethical practices. A granular analysis of individual company conduct is essential to dissect the specific nature of these commitments. South African pharmaceutical corporations display a mean value superior to Russia, India, and China but inferior to Brazil. The notably high standard deviation signifies a broad spectrum of bioethical considerations within these companies, highlighting the imperative for an all-encompassing understanding of individual company policies. This analysis underscores the imperative of understanding the nuanced nature of bioethical commitments within each BRICS nation and highlights the cardinal importance of these considerations in the pharmaceutical industry. The research augments our comprehension of the specific ethical priorities in these nations, offering crucial insights for future research and policy development, which could considerably influence global bioethics standards.
Keywords: Bioethics; Pharmaceutical; BRICS; Ethical; Standards.
Introduction
Bioethical standards, as meticulously outlined by the World Health Organization (WHO), serve as a paramount framework that underpins ethical decision-making within the multifaceted healthcare sector [1]. These standards encompass an expansive array of ethical principles, encompassing, but by no means limited to, the fundamental tenets of patient autonomy, transparency, accountability, and equitable access to healthcare services and products. These principles collectively embody the core values of the healthcare profession, weaving a complex tapestry of ethical considerations that address the intricate interplay of health, medicine, and society. The WHO’s bioethical guidelines are thoughtfully designed to ensure that healthcare organizations, professionals, and institutions place ethical considerations at the forefront of their operations and decisionmaking processes [2]. This is not a mere suggestion but a moral imperative to safeguard the welfare and dignity of individuals, communities, and societies at large. The guidelines advocate for patient-centered care that respects individual choices and upholds the principles of beneficence and non-maleficence. They advocate for transparency, ensuring that information and decisions are clear and comprehensible, fostering trust and shared decision-making [3]. Furthermore, accountability is an essential pillar, reminding healthcare entities of their responsibilities to patients, communities, and the broader public. Equitable access to healthcare services and products is a fundamental commitment, reflecting the belief that healthcare should be a universal right, not a privilege. In essence, the WHO’s bioethical guidelines set the ethical compass for healthcare, reflecting a commitment to values that transcend borders and cultures, guiding the global community towards a more ethical and equitable healthcare landscape [4].
The pharmaceutical industry, with its profound impact on global health, plays a pivotal role in implementing and upholding these bioethical standards. As manufacturers of life-saving drugs and medical interventions, pharmaceutical companies bear a significant ethical responsibility. Their decisions influence not only the well-being of patients but also the broader healthcare landscape, shaping access to medicines, research practices, and the equitable distribution of healthcare resources [5].
The BRICS nations - Brazil, Russia, India, China, and South Africa - have emerged as notable players in the global pharmaceutical sector. These nations host some of the world’s largest pharmaceutical companies and contribute substantially to the development and production of pharmaceutical products. Understanding the bioethical commitments of these leading pharmaceutical companies within the BRICS nations is critical in evaluating their dedication to responsible and ethical pharmaceutical practices [6].
This research study is motivated by the need to assess and compare the bioethical orientations of the top pharmaceutical companies in the BRICS countries. Bioethical themes and principles [7], as evident in the vision and mission statements of these companies, provide a lens through which to evaluate their commitment to responsible and ethical pharmaceutical practices. A content analysis [8] of these statements enabled the identification of nuances in how these organizations publicly express their dedication to bioethical standards. Additionally, this research seeks to determine which BRICS nation excels in integrating bioethical considerations within its pharmaceutical industry, shedding light on regional dynamics [9].
Research objectives
This study’s primary objectives are as follows:
1. To analyze the vision and mission statements of the top pharmaceutical companies in the BRICS nations to identify and understand the bioethical themes and principles they emphasize.
2. To compare and contrast the extent to which these companies commit to bioethical standards, discerning potential variations in their dedication to ethical pharmaceutical practices.
3. To ascertain which of the BRICS nations stands out as a leader in incorporating bioethical considerations within its pharmaceutical industry.
Literature review
In the realm of public health research and surveillance, the guidelines established by the World Health Organization (WHO) serve as a vital framework for ethical and effective practices. These guidelines provide essential principles that guide the development, implementation, and management of public health surveillance systems, ensuring that they not only safeguard individual rights but also contribute to the broader well-being of communities and nations. This literature section delves into a comprehensive analysis of WHO’s Ethical Issues in Public Health Surveillance guidelines, exploring their critical components and the imperative role they play in research. Each guideline elucidates a set of fundamental principles, ranging from the development of surveillance systems tailored to specific contexts in research [10].
The literature review section initiates the study, exploring the existing body of knowledge on bioethics within the pharmaceutical industry. It places specific emphasis on the global context and the bioethical guidelines delineated by the WHO. The section underscores the central role played by these guidelines in shaping ethical healthcare practices and provides the necessary context for the subsequent analysis.
An in-depth examination of the bioethical standards outlined by the WHO follows, providing the foundational framework for evaluating the bioethical content present within the vision and mission statements of the selected pharmaceutical companies. The principles set forth by the WHO, which include patient autonomy, beneficence, non-maleficence, justice, transparency, and accountability, serve as a benchmark against which the companies will be evaluated. These principles constitute the ethical compass that guides the actions and decisions of pharmaceutical companies, making them central to the methodology of this study.
Table 1: WHO bioethical guiding principles.
| WHO bioethical guiding principles | Contribution | ||
|---|---|---|---|
| Development | Transparent | Vulnerable Populations |
Communication |
| Mechanisms | Support | Data Security | Emergency |
| Legitimate | Community Engagement | Data Sharing | Research |
| Quality | Risk Management | Justification | Protection |
In the course of this research, the WHO’s 17 ethical guidelines on Public Health Surveillance have been adopted as the primary framework [11]. These guidelines have been instrumental in ensuring the ethical soundness of the study, offering a comprehensive roadmap for addressing complex ethical issues that often arise in public health surveillance. Table 1 provides an at-a-glance representation of the 17 WHO guideline keywords, which were carefully extracted from the extensive WHO guidelines. This visualization offers a succinct overview of the key ethical principles guiding the study, making it easier for readers to grasp the foundation upon which this research is built.
Development
In adherence to Guideline 1, countries are ethically bound to establish public health surveillance systems that are not only appropriate for their unique contexts but also feasible and sustainable over the long term. These systems should serve a distinct and well-defined purpose, encompassing a strategic blueprint for data collection, analysis, utilization, and distribution, all of which must be intricately linked to the most pressing public health priorities. This foundational guideline underscores the critical importance of having effective and ethically-driven public health surveillance systems that contribute to the protection and improvement of public health on both a national and global scale [11].
Mechanism
In accordance with Guideline 2, nations bear the responsibility of creating ethical public health surveillance systems that not only meet the requirements of appropriateness but are also underpinned by effective mechanisms. These mechanisms serve as the ethical bedrock of surveillance, ensuring that the process is conducted with a strong focus on safeguarding privacy, upholding human rights, and fostering trust within the community. Ethical surveillance, as per this guideline, necessitates not only the establishment of systems that collect and manage data with the utmost integrity but also the implementation of policies, guidelines, and practices that protect the interests and rights of individuals and communities. By emphasizing the need for ethical mechanisms, Guideline 2 reinforces the vital balance between public health interests and individual rights, thereby promoting the responsible and transparent use of surveillance data [12].
Legitimate
Guideline 3 underscores the fundamental principle that surveillance data should be gathered exclusively for legitimate public health purposes. In adherence to this ethical imperative, public health surveillance activities should be grounded in clear and specific objectives that prioritize the welfare of the population. Any data collection, whether it involves disease tracking, risk assessment, or health intervention planning, must be directly linked to the overarching goal of safeguarding and improving public health. This guideline is instrumental in steering surveillance practices away from intrusive or unnecessary data collection, serving as a protective barrier against potential violations of privacy or misuse of information. By ensuring that data collection is firmly aligned with the overarching objective of promoting public health, Guideline 3 upholds the ethical standards essential for responsible and effective surveillance [13].
Quality
Guideline 4 highlights the crucial responsibility of nations in guaranteeing that surveillance data meet stringent quality standards. This involves ensuring data are not only collected in a timely and consistent manner but also that they maintain a high degree of reliability and validity. Timeliness ensures that data is up-to-date and can inform swift responses to emerging public health challenges. Reliability means that the data is consistent and reproducible, contributing to the robustness of findings. Validity assures that the data accurately reflect the public health phenomena under scrutiny. These qualities collectively form the bedrock of effective surveillance systems, permitting the informed decision-making necessary for achieving public health objectives and addressing health threats in a precise and accountable manner [14,11].
Transparent
Guideline 5 stresses the significance of a transparent approach to planning public health surveillance, emphasizing the central role of governmental priority-setting. This involves an open and inclusive process where priorities are defined, objectives are established, and resources are allocated in a clear and accountable manner. Transparent priority-setting ensures that public health surveillance efforts are aligned with the most pressing health concerns and that decision-making processes are accessible to the public. This transparency is essential for garnering public trust, fostering collaboration, and promoting ethical practices in surveillance planning and execution, ultimately enhancing the effectiveness of these systems in safeguarding public health [15].
Support
Guideline 6 underscores the global community’s moral obligation to assist nations lacking sufficient resources to carry out surveillance effectively [16]. This obligation emphasizes international collaboration to bridge resource gaps, enabling these countries to implement and maintain surveillance systems that contribute to global public health security [17].
Community engagement
Guideline 7 emphasizes the imperative consideration of the values and concerns of communities throughout the entire process of public health surveillance, encompassing planning, implementation, and data utilization. This directive promotes a people-centered approach, ensuring that surveillance activities are conducted with respect for cultural, ethical, and societal norms, and that the rights and privacy of individuals are upheld. It underscores the significance of engaging communities in decision-making and data use, fostering trust and accountability while aligning surveillance practices with the best interests of the populations being monitored [18].
Risk management
Guideline 8 outlines the critical steps that individuals responsible for surveillance should follow to ensure ethical and responsible practices. It mandates the identification, evaluation, minimization, and disclosure of potential risks for harm before surveillance activities are initiated [19]. Furthermore, this guideline necessitates continuous monitoring to promptly detect any harm that may arise during the surveillance process. In the event of identified harm, it calls for the immediate implementation of appropriate measures to mitigate and address the adverse consequences, thereby upholding the principles of surveillance that prioritize the welfare and rights of those under surveillance [20,21].
Vulnerable populations
Guideline 9 emphasizes the vital importance of surveillance for individuals or groups particularly vulnerable to disease, harm, or injustice. It calls for careful scrutiny and ethical consideration to prevent the imposition of unnecessary additional burdens on these vulnerable populations. This guideline underscores the ethical responsibility to protect the rights and wellbeing of those who are more susceptible, ensuring that surveillance activities do not exacerbate their vulnerabilities but instead contribute to their protection and equitable access to healthcare and public health services [23].
Data security
Guideline 10 directs governments and data custodians to prioritize the secure management of identifiable data obtained through surveillance. It underscores the critical responsibility to establish robust security measures to protect these data from unauthorized access, breaches, or misuse. Ensuring the appropriate safeguarding of identifiable data is essential to uphold privacy, trust, and ethical practices in public health surveillance, guaranteeing that sensitive information remains confidential and that individuals’ rights are respected [23].
Justification
Guideline 11 acknowledges that, in specific situations, the collection of names or identifiable data is ethically justified. It highlights that such collection should be carefully considered and warranted based on the public health goals and objectives of the surveillance. This guideline recognizes that there are instances where the collection of identifiable data is essential for targeted and effective public health responses while emphasizing the importance of transparency and responsible use of this data in these circumstances [24,25].
Contribution
Guideline 12 underscores the obligation of individuals to participate in surveillance when their contribution provides reliable, valid, and complete data sets, and when relevant protection measures are in place. In such circumstances, this guideline recognizes that the requirement for informed consent is ethically waived, acknowledging that the public good and the protection of public health take precedence over individual consent. It underscores the balance between individual rights and the collective responsibility to support effective surveillance practices [26].
Communication
Guideline 13 stresses the necessity of efficiently communicating the results of surveillance to pertinent target audiences. It underlines the importance of delivering findings, insights, and relevant information to individuals, communities, and organizations that can act on or benefit from the data. This guideline promotes transparency and accountability in public health surveillance, ensuring that stakeholders and the wider community are informed and empowered to make informed decisions based on the surveillance results [27].
Data sharing
Guideline 14 emphasizes that individuals and entities responsible for public health surveillance have a moral obligation to share data with other national and international public health agencies when it is supported by appropriate safeguards and justification [11]. This guideline underscores the importance of collaboration and information exchange to address global health challenges effectively. It calls for responsible sharing of data to support the collective efforts of public health agencies, promoting transparency and collective action in the face of health threats [28].
Emergency
Guideline 15 highlights the critical imperative that all parties engaged in surveillance promptly share data during a public health emergency. It underscores the urgency and necessity of rapid information exchange to facilitate an effective and coordinated response to emerging health crises. This guideline emphasizes the vital role of timely data sharing in preventing, mitigating, and controlling public health emergencies, fostering a collaborative approach among stakeholders [29].
Research
Guideline 16 outlines that, with proper justification and safeguards in place, public health agencies have the authority to use or share surveillance data for research purposes. It underscores the importance of ethical and responsible use of data to advance scientific understanding and contribute to public health knowledge. This guideline promotes the responsible conduct of research within the framework of public health surveillance, prioritizing the protection of individual rights and privacy [30].
Protection
Guideline 17 stresses that personally identifiable surveillance data should not be disclosed to agencies that may exploit the data to take adverse actions against individuals or for purposes unrelated to public health. It underscores the importance of safeguarding individual rights and privacy by preventing the misuse of sensitive information. This guideline prioritizes the ethical and responsible use of personal data, ensuring it is solely employed for legitimate public health purposes and not for detrimental or unrelated actions [31].
BRICS institutes addressing bioethical dilemmas
In the dynamic landscape of the BRICS nations, several institutions are at the forefront of examining and addressing bioethical dilemmas. These organizations play a pivotal role in navigating the complex intersection of rapidly advancing biotechnology, healthcare practices, and cultural contexts within Brazil, Russia, India, China, and South Africa.
In Brazil, the Center for Bioethics and Culture stands as a prominent institution devoted to bioethical research and public awareness. Operating at the crossroads of academia and society, the center engages in interdisciplinary research, fostering dialogue on bioethical challenges unique to the Brazilian context. Its initiatives include organizing conferences, seminars, and educational programs, promoting ethical considerations in biomedicine and healthcare practices.
The Institute of Philosophy at the Russian Academy of Sciences takes a leading role in exploring the ethical dimensions of biomedicine and biotechnology in Russia. Comprising scholars from diverse disciplines, the institute conducts research, publishes scholarly articles, and organizes conferences to address the ethical implications of scientific advancements. Its multidisciplinary approach contributes significantly to the understanding and resolution of bioethical issues within the Russian Federation.
India:
India’s Forum for Medical Ethics Society is a key player in promoting ethical practices in healthcare. Committed to fostering awareness and understanding of bioethical concerns, the forum conducts workshops, seminars, and training programs for healthcare professionals and the general public. Through its publications and collaborations, the society actively contributes to shaping ethical discourse and guidelines in the Indian healthcare landscape.
In China, the Center for Bioethics at Huazhong University of Science and Technology plays a vital role in research and education in bioethics. The center focuses on ethical considerations in biomedicine, biotechnology, and healthcare delivery. Through academic research projects, training programs, and collaborations with international institutions, the center contributes to the ethical development of China’s rapidly advancing biomedical and technological sectors.
The Steve Biko Centre for Bioethics in South Africa is a significant institution addressing ethical challenges related to health, human rights, and social justice. The center conducts research, provides training, and engages in community outreach programs to promote ethical practices in healthcare. By integrating perspectives from diverse communities, the center contributes to the development of ethically informed policies and practices in South Africa’s healthcare system.
These institutions within the BRICS nations actively contribute to the global conversation on bioethics, each with a unique approach shaped by its national context, cultural nuances, and commitment to fostering ethical practices in science, medicine, and healthcare.
Methodology
The research methodology adopted for this study is designed to provide a comprehensive and systematic analysis of bioethical considerations in the vision and mission statements of leading pharmaceutical companies listed in the stock exchanges of BRICS nations. The BRICS nations, comprising Brazil, Russia, India, China, and South Africa, represent emerging economic powers, and their pharmaceutical industries are critical players in the global healthcare landscape. To ensure a robust and representative analysis, the following detailed steps were undertaken:
Selection of pharmaceutical companies
To ensure a comprehensive overview of the pharmaceutical industry in BRICS countries, the top five publicly listed pharmaceutical companies were selected in each country. These companies were chosen based on their market capitalization, reputation, and prominence in their respective countries’ pharmaceutical sectors.
Data source
The primary data source for this analysis was the official vision and mission statements of the selected pharmaceutical companies. These statements are publicly accessible and provide valuable insights into the core values, objectives, and bioethical stances of these companies. Vision and mission statements are integral to a company’s identity and convey its commitment to various stakeholders, including customers, investors, and the public.
Thematic analysis
Thematic analysis was employed to identify recurring patterns, themes, and keywords within the vision and mission statements. The goal was to extract and categorize bioethical content within these statements. Bioethical considerations encompass a wide range of topics, such as patient welfare, ethical drug development, transparency, access to medicines, and corporate social responsibility.
Bioethical principles
To evaluate the presence and prominence of bioethical content, the analysis was guided by the bioethical principles defined by the World Health Organization (WHO). WHO’s guidelines on bioethics provide a comprehensive framework that covers diverse aspects of ethical practices in the healthcare and pharmaceutical industries. These principles served as a benchmark against which the content within the statements was assessed.
Comparative analysis
After extracting the thematic content and identifying key bioethical keywords, a comparative analysis was conducted to assess how well the selected pharmaceutical companies aligned with WHO’s bioethical guidelines. This analysis aimed to identify the extent to which these companies prioritize and emphasize bioethical considerations in their mission and vision statements.
Identification of influential BRICS nation
The study culminated in a comparative assessment to determine which BRICS nation, based on the vision and mission statements of its pharmaceutical companies, exhibited the highest degree of alignment with WHO’s bioethical principles. This comparative analysis provides valuable insights into the ethical commitment of pharmaceutical companies and the national regulatory environments in which they operate.
By rigorously following these steps, this research method seeks to offer a nuanced understanding of the bioethical landscape within the pharmaceutical industry of BRICS nations. It identifies key players in each country, their ethical priorities, and the extent to which they align with global bioethical standards set by the WHO. This methodology lays the foundation for an in-depth examination of the pharmaceutical industry’s ethical practices and their potential implications for public health and global bioethics standards.
Data analysis
In line with the established methodology, the data analysis section of this study is designed to systematically evaluate the bioethical commitments of the top pharmaceutical companies in the BRICS nations, guided by the ethical principles delineated by the World Health Organization (WHO). This analysis centers on the vision and mission statements of these companies, which represent the public articulation of their core values and objectives. The initial step involves data preparation, wherein the vision and mission statements of the top five publicly listed pharmaceutical companies from each BRICS country are collected and organized. Thematic analysis is then employed to uncover recurring patterns, themes, and keywords within these statements, with a specific focus on bioethical considerations such as patient welfare, ethical drug development, transparency, access to medicines, and corporate social responsibility. The bioethical principles established by WHO serve as the benchmark against which the identified themes and keywords are coded, providing a structured framework for assessment. The subsequent steps involve the quantitative assessment of the prominence of bioethical content within these statements and a comparative analysis to discern which BRICS nation demonstrates the highest degree of alignment with global bioethical standards. Ultimately, this data analysis endeavors to offer a comprehensive understanding of the ethical landscape within the pharmaceutical industry of BRICS nations, shedding light on the prioritization of bioethical considerations and their potential impact on global bioethics standards.
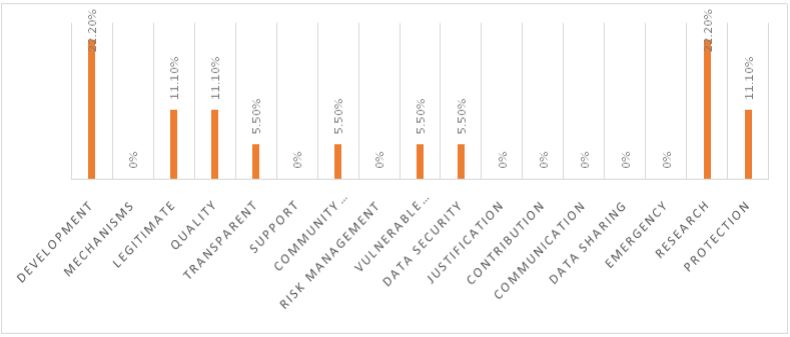
WHO guidelines highlighted by Brazil
In Figure 1, the analysis of the keyword frequencies in the vision and mission statements of Brazilian pharmaceutical companies, in the context of WHO guidelines for pharmaceutical companies, reveals significant insights into their core principles and areas of focus. Two keywords, “Development” and “Research,” both stand out prominently with a substantial frequency of 22.20%, underscoring these companies’ strong commitment to advancing pharmaceutical products and services in line with WHO guidelines. This commitment aligns with WHO’s emphasis on innovation, accessibility, and the advancement of scientific knowledge, all of which are crucial factors for enhancing global health and the quality of healthcare interventions. The absence of the keyword “Mechanisms” in the statements implies that while specific operational mechanisms may not be explicitly mentioned, they are likely implied within the companies’ activities, demonstrating that the how of their operations is not extensively detailed in the missions. “Legitimate” is mentioned at a frequency of 11.10%, reflecting these organizations’ dedication to conducting pharmaceutical operations in an ethical and lawful manner, a key component of WHO’s guidelines promoting ethical and legitimate practices in the pharmaceutical industry. Similarly, the emphasis on “Quality” at the same frequency aligns with WHO guidelines, which prioritize the production of high-quality pharmaceutical products and services to ensure the safety and efficacy of healthcare interventions. The mention of “Transparent” at 5.50% underscores the commitment to transparency, in line with WHO’s principles that advocate for transparency in the pharmaceutical industry, which is essential for building trust with stakeholders and ensuring ethical and responsible pharmaceutical operations. “Community Engagement,” “Vulnerable Populations,” and “Data Security” each have a frequency of 5.50%. These keywords highlight the companies’ dedication to engaging with communities, addressing the needs of vulnerable populations, and protecting sensitive data, all of which are in alignment with various aspects of WHO guidelines related to ethical and responsible pharmaceutical practices. However, other keywords like “Support,” “Risk Management,” “Justification,” “Contribution,” “Communication,” “Data Sharing,” “Emergency,” and “Mechanisms” all have a frequency of 0%, indicating that they may not be central themes in the missions of the Brazilian pharmaceutical companies. This suggests that these specific aspects are not explicitly emphasized in the vision and mission statements, or they may be addressed under broader, more general terms that are not reflected in these keywords.
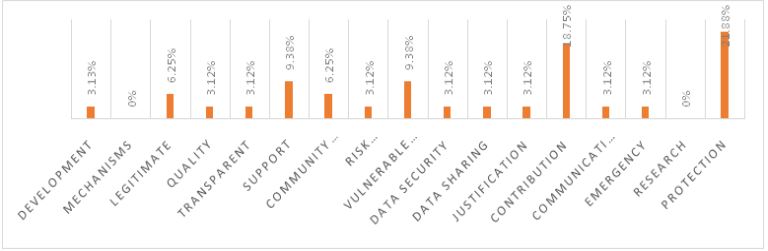
WHO guidelines highlighted by Russia
The analysis of the top 5 nationally listed pharmaceutical companies in Russia, in the context of the WHO’s 17 guidelines for pharmaceutical companies in Figure 2, reveals several important insights. It is evident that these companies are predominantly focused on protection (21.88%) and contribution (18.75%), underscoring their commitment to ensuring product safety and making a significant societal impact. However, the absence of any mention of ‘research’ (0%) in their vision and mission statements is a notable gap, highlighting a potential area for improvement in terms of their dedication to scientific advancements and innovation. Furthermore, the low percentages in areas such as development (3.13%), quality (3.12%), and transparency (3.12%) suggest that there may be room for enhancing their commitment to these critical aspects of pharmaceutical operations. The emphasis on vulnerable populations (9.38%) and community engagement (6.25%) indicates a strong commitment to social responsibility and ethical considerations in their operations. The inclusion of ‘data security’ and ‘data sharing’ (both at 3.12%) in their statements reflects a growing awareness of the importance of data ethics in the pharmaceutical industry. In conclusion, the vision and mission statements of Russian pharmaceutical companies demonstrate a strong commitment to ethical considerations, particularly in terms of protection, contribution, and social responsibility. However, they should aim to address the lack of focus on research and development while also increasing their attention to areas like development, quality, and transparency to better align with the comprehensive scope of WHO guidelines for bioethical practices. This holistic approach is not only crucial for meeting global standards but also for strengthening their position within the BRICS nations.
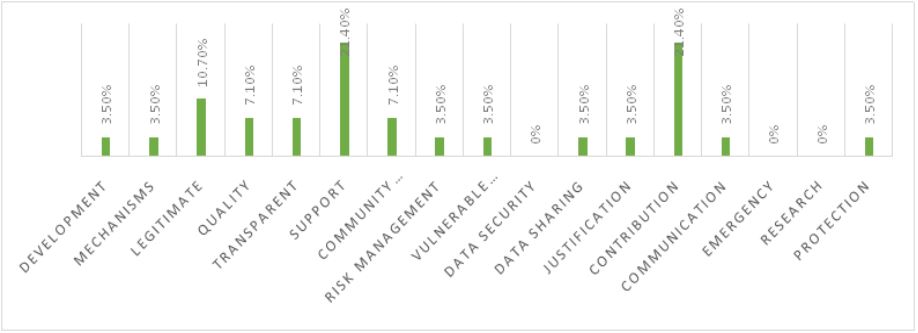
WHO guidelines highlighted by India
The analysis of the top 5 pharmaceutical companies in India, based on their vision and mission statements, in the context of the WHO’s 17 guidelines for pharmaceutical companies, provides valuable insights. It is evident that these Indian companies place a strong emphasis on support (21.40%) and contribution (21.40%), showcasing their commitment to providing assistance and making a significant positive impact. The focus on legitimate operations (10.70%) further underscores their dedication to ethical and responsible practices. Additionally, the attention given to quality (7.10%), transparency (7.10%), and community engagement (7.10%) reflects a comprehensive commitment to ethical and responsible business practices in the pharmaceutical sector.
However, certain areas deserve attention. The complete absence of any reference to ‘data security’ and ‘emergency’ (both 0%) in their vision and mission statements suggests a potential gap in addressing these important aspects. Moreover, ‘research’ and ‘mechanisms’ both received relatively low percentages (3.50%), implying the need for a more significant focus on research and development activities and regulatory compliance mechanisms. Furthermore, ‘vulnerable populations,’ ‘data sharing,’ ‘justification,’ and ‘protection’ also received 3.50%, indicating a balanced commitment across these aspects.
The vision and mission statements of Indian pharmaceutical companies demonstrate a strong commitment to ethical considerations, particularly in terms of support, contribution, legitimacy, quality, transparency, and community engagement. However, they should work on strengthening their approach to data security, emergency preparedness, research and development, and specific aspects like data sharing, justification, and protection, to align more closely with the complete spectrum of WHO guidelines for bioethical practices. This comprehensive alignment is vital not only for adhering to global standards but also for enhancing their position within the pharmaceutical industry, and further contributing to the BRICS nations’ healthcare landscape.
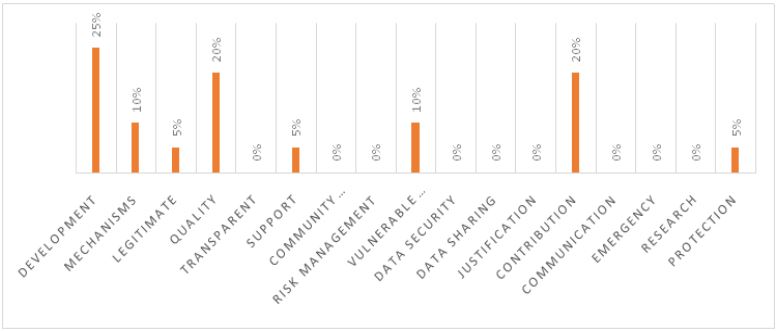
WHO guidelines highlighted by China
The analysis of the top pharmaceutical companies in China, based on their vision and mission statements, in the context of the WHO’s 17 guidelines for pharmaceutical companies, provides important insights. Chinese pharmaceutical companies place a significant emphasis on development (25%) and quality (20%), showcasing their commitment to research and the production of high-quality medicines. Additionally, they demonstrate a substantial focus on mechanisms (10%) and vulnerable populations (10%), which implies a commitment to efficient operational processes and ethical considerations for underserved communities.
However, there are notable gaps in some areas. The complete absence of any reference to ‘transparent,’ ‘community engagement,’ ‘risk management,’ ‘data security,’ ‘data sharing,’ ‘justification,’ ‘communication,’ ‘emergency,’ and ‘research’ (all at 0%) in their vision and mission statements indicates the potential for improvement in addressing these crucial aspects. Furthermore, ‘legitimacy’ and ‘protection’ both received relatively low percentages (5%), suggesting the need for a more robust commitment to ethical and regulatory standards in their business practices.
The vision and mission statements of Chinese pharmaceutical companies reflect a strong commitment to research, development, and ensuring the quality of medicines. However, there is room for improvement in terms of transparency, community engagement, risk management, data security, data sharing, justification, communication, emergency preparedness, and research activities. A more comprehensive alignment with the complete spectrum of WHO guidelines for bioethical practices is crucial not only for adhering to global standards but also for enhancing their position within the pharmaceutical industry, and contributing to the healthcare landscape of the BRICS nations.
WHO guidelines highlighted by South Africa
The analysis of the top pharmaceutical companies in South Africa, based on their vision and mission statements, in the context of the WHO’s 17 guidelines for pharmaceutical companies, provides valuable insights. South African pharmaceutical companies demonstrate a particularly strong emphasis on legitimacy (35%) and contribution (22.80%), highlighting their commitment to ethical and responsible practices, and making a significant societal impact. Quality (19.20%) and support (8.70%) also receive significant attention, reflecting a comprehensive approach to delivering high-quality medicines and providing assistance.
However, there are certain areas that require attention. The absence of any reference to ‘community engagement,’ ‘risk management,’ ‘data security,’ ‘data sharing,’ ‘justification,’ ‘emergency,’ and ‘research’ (all at 0%) in their vision and mission statements suggests potential gaps in addressing these crucial aspects. Additionally, the relatively low percentages for ‘transparent’ (1.70%), ‘vulnerable populations’ (1.70%), and ‘communication’ (1.70%) imply a need for a more pronounced focus on transparency, engagement with underserved communities, and effective communication strategies.
The vision and mission statements of South African pharmaceutical companies reflect a strong commitment to ethical considerations, particularly in terms of legitimacy, contribution, quality, and support. However, they should work on strengthening their approach to transparency, community engagement, risk management, data security, data sharing, justification, emergency preparedness, and research. This comprehensive alignment with the complete spectrum of WHO guidelines for bioethical practices is not only essential for adhering to global standards but also for enhancing their position within the pharmaceutical industry, and further contributing to the healthcare landscape of the BRICS nations.
Overall WHO guidelines highlighted by BRICS countries
In Table 2 the details for Brazil’s pharmaceutical companies have a mean value of 7.1, which is the highest among the BRICS nations. This suggests that these companies place a substantial emphasis on bioethical considerations in their vision and mission statements. However, the standard deviation of 7.8 is also the highest, indicating a wide range of variability in their approach to bioethics. This variance could reflect the diversity among Brazilian pharmaceutical companies, with some being more committed to bioethical practices than others. Further exploration of individual company practices and policies is necessary to understand the specific nature of these bioethical considerations. Despite the variation, the overall commitment to bioethical practices in Brazil’s pharmaceutical sector is notable.
Russian pharmaceutical companies have a mean value of 2.6, indicating a relatively lower emphasis on bioethical considerations compared to Brazil. The standard deviation of 2.8 suggests moderate variation in the bioethical commitments of these companies. While they may not prioritize bioethics to the same extent as Brazilian counterparts, the moderate standard deviation indicates that there is some level of diversity in bioethical practices among Russian pharmaceutical companies. Further analysis is required to understand the specific factors influencing their approach to bioethics, and whether there are opportunities for greater alignment with global bioethical standards.
Table 2: Overall WHO guidelines highlighted by BRICS countries
| Country | Mean | Standard Deviation |
|---|---|---|
| Brazil | 7.1 | 7.8 |
| Russia | 2.6 | 2.8 |
| India | 1.3 | 1.6 |
| China | 1.7 | 1.7 |
| South Africa | 4.2 | 5.1 |
Indian pharmaceutical companies exhibit the lowest mean value among the BRICS nations, with a mean of 1.3. This suggests that bioethical considerations may not be as central in their vision and mission statements as in other countries. The standard deviation of 1.6 indicates moderate variation, implying that while the mean is low, there is still diversity in bioethical practices among Indian pharmaceutical companies. A comprehensive exploration of the specific bioethical commitments of these companies is needed to understand the extent to which they prioritize ethical considerations in their operations and products. This analysis may uncover opportunities for enhanced bioethical practices in the Indian pharmaceutical sector.
Chinese pharmaceutical companies have a mean value of 1.7, which is slightly higher than India but still reflective of a relatively lower emphasis on bioethical considerations. Interestingly, the standard deviation of 1.7 matches the mean, suggesting a degree of uniformity in bioethical practices among Chinese pharmaceutical companies. While their overall commitment to bioethics may be lower, the consistency in their approach indicates a standardized stance on ethical considerations. Detailed examination of individual company practices is necessary to assess the specific nature of their bioethical commitments and identify areas for improvement.
South African pharmaceutical companies have a mean value of 4.2, which is higher than Russia, India, and China but lower than Brazil. The standard deviation of 5.1 is the highest among the BRICS nations, signifying a broad range of bioethical considerations within South African pharmaceutical companies. This diversity suggests that while some companies prioritize bioethical practices to a considerable extent, others may have a less prominent focus on bioethics. Further investigation into the specific bioethical commitments of South African companies is essential to understand the factors contributing to this diversity. It is evident that bioethics is a significant aspect of the pharmaceutical sector in South Africa, but the variation indicates the need for tailored strategies to ensure consistent adherence to ethical standards.
The analysis reveals significant variations in the emphasis on bioethical considerations in the vision and mission statements of leading pharmaceutical companies in BRICS nations. Brazil stands out with the highest mean, while the other countries demonstrate varying degrees of commitment to bioethics. The standard deviation values highlight the range and diversity within each country, calling for a more comprehensive examination of specific company practices and policies. This detailed analysis underscores the importance of understanding the specific nature of bioethical commitments within each BRICS nation and provides valuable insights for future research and policy development.
Most highlighted WHO guidelines by BRICS
Brazil’s pharmaceutical sector places a significant emphasis on the WHO guidelines related to “Development” and “Research.” This suggests a strong commitment to advancing healthcare research and development within the country. Brazil is likely prioritizing initiatives that contribute to the growth and improvement of the pharmaceutical industry and the healthcare sector. This focus aligns with the goal of enhancing access to quality healthcare and innovative medical solutions.
Russia’s pharmaceutical industry centers its attention on the WHO guideline of “Protection.” This could imply a strong emphasis on safeguarding various aspects of pharmaceutical operations, including patient safety, data privacy, and intellectual property protection. By prioritizing protection, Russian pharmaceutical companies are likely striving to ensure the integrity and security of their products and operations.
India’s pharmaceutical sector shows a primary focus on the WHO guideline of “Support.” This suggests that Indian pharmaceutical companies may prioritize initiatives that offer support to various stakeholders, including patients, healthcare professionals, and the broader community. Supportive measures could include affordable access to medicines, medical assistance programs, and collaborations aimed at addressing healthcare challenges.
China’s pharmaceutical industry emphasizes the WHO guideline of “Development.” This focus aligns with China’s commitment to advancing its healthcare infrastructure, research capabilities, and pharmaceutical innovation. It signifies a concerted effort to foster growth and development within the healthcare and pharmaceutical sectors. China may be investing in research, education, and technological advancements to achieve its development goals.
South Africa’s pharmaceutical sector underscores the WHO guideline of “Legitimate.” This focus suggests a strong commitment to adhering to legitimate and ethical business practices in the pharmaceutical industry. South African pharmaceutical companies are likely prioritizing transparency, integrity, and ethical conduct in their operations. This focus is crucial for building trust and ensuring the legitimacy of pharmaceutical products and services.
Table 3: Most highlighted and WHO guidelines by BRICS.
| Country | WHO main focused Guideline |
|---|---|
| Brazil | Development and Research |
| Russia | Protection |
| India | Support |
| China | Development |
| South Africa | Legitimate |
The systematic analysis of the WHO main focused guidelines in BRICS countries’ pharmaceutical practices provides valuable insights into their priorities and commitments. Each country’s emphasis on specific WHO guidelines reflects its unique approach to pharmaceutical business, aligned with its national goals and healthcare landscape. Understanding these focal points can guide future research and policymaking, as well as promote collaboration and knowledge sharing among BRICS nations to further enhance pharmaceutical practices and healthcare outcomes.
The examination of BRICS institutions addressing bioethical dilemmas illuminates the critical role played by these entities in shaping the ethical landscape of their respective nations. Notably, the Center for Bioethics and Culture in Brazil emerges as a pivotal institution engaging in interdisciplinary research and fostering dialogue on bioethical challenges unique to the Brazilian context. The study findings resonate with the emphasis on bioethical considerations within Brazil’s pharmaceutical sector, revealing a substantial commitment reflected in the highest mean value. Similarly, the Institute of Philosophy in Russia, highlighted in the analysis, contributes significantly to exploring the ethical dimensions of biomedicine and biotechnology within the nation. The study’s revelation of a relatively lower emphasis on bioethical considerations in Russia aligns with the emphasis on “Protection” within the vision and mission statements of Russian pharmaceutical companies. The Forum for Medical Ethics Society in India, identified as a key player in promoting ethical practices, is mirrored in the study findings that suggest a lower mean value and moderate variation in bioethical commitments among Indian pharmaceutical companies. Meanwhile, the Center for Bioethics in China, noted for its role in research and education, corresponds to the study’s indication of a relatively lower emphasis on bioethical considerations in Chinese pharmaceutical companies. Lastly, the Steve Biko Centre for Bioethics in South Africa, highlighted for addressing ethical challenges, aligns with the study’s findings of a diverse range of bioethical considerations within South African pharmaceutical companies, emphasizing the need for an all-encompassing understanding of individual company policies. In essence, these BRICS institutions are integral to not only fostering dialogue and shaping ethical discourse but also influencing the bioethical commitments of the pharmaceutical industry in alignment with the specific contexts and priorities of their respective nations, as revealed by the study’s detailed analysis.
Implication of study and future research
The research on Bioethical Considerations in Leading Pharmaceutical Companies has revealed valuable insights into the bioethical landscape within the pharmaceutical industry of BRICS nations. The analysis, based on the vision and mission statements of the top pharmaceutical companies in Brazil, Russia, India, China, and South Africa, offers significant implications for the field of bioethics and provides a foundation for future research endeavors. The study highlights diverse bioethical priorities, with Brazil standing out as a leader in bioethical commitment, emphasizing development and research. This diversity has implications for collaboration and knowledge sharing among BRICS countries to promote best practices in healthcare and pharmaceutical ethics. The study also underscores the importance of ethical practices in the pharmaceutical industry, as companies that prioritize bioethical considerations are likely to build trust with stakeholders, including patients and investors. Emphasis on protection, support, and legitimacy aligns with the broader goal of fostering ethical conduct. The comparative analysis provides insights into the national regulatory environments in which pharmaceutical companies operate and their potential influence on global bioethical standards set by the World Health Organization (WHO). Future research can explore in-depth company analysis, conduct comparative studies, focus on policy development, assess long-term impacts of bioethical practices, and investigate the effectiveness of ethical education and training for healthcare professionals. This research contributes to the promotion of public health, the alignment with global bioethics standards, and the enhancement of ethical practices in the pharmaceutical industry, ultimately benefiting society and patients.
These highlighted BRICS institutions, recognized for their significant contributions to addressing bioethical dilemmas, can further strengthen their impact through several strategic recommendations. Firstly, fostering increased international collaboration will enable these institutions to share best practices, methodologies, and research outcomes, enriching the global discourse on bioethics. Secondly, instituting context-specific training programs will empower professionals within their nations, ensuring a deeper understanding of the unique bioethical challenges in the pharmaceutical sector. Additionally, active engagement in policy advocacy at both national and international levels will amplify the influence of these institutions in shaping ethically informed policies. Enhancing transparency and reporting standards within pharmaceutical companies, supported by these institutions, will contribute to more comprehensive disclosure of bioethical practices. Lastly, continual monitoring and evaluation mechanisms can be implemented to track adherence to ethical guidelines and assess the effectiveness of institutional initiatives over time. Through these measures, these BRICS institutions can collectively foster a more ethically robust pharmaceutical industry in alignment with the specific contexts and priorities of their respective nations.
Conclusion
In conclusion, the research on “Bioethical Considerations in Leading Pharmaceutical Companies: A Comparative Analysis in BRICS Nations” provides valuable insights into the bioethical landscape within the pharmaceutical industry of Brazil, Russia, India, China, and South Africa. The analysis of vision and mission statements of top pharmaceutical companies reveals significant variations in the emphasis on bioethical considerations among these BRICS nations. Brazil stands out with the highest mean, indicating a substantial commitment to bioethics, albeit with notable diversity among its companies. Russia, India, and China exhibit varying degrees of bioethical commitment, with differences in their standard deviations highlighting diversity in their approaches. South Africa, while demonstrating a noteworthy emphasis on bioethics, also exhibits a wide range of considerations within its pharmaceutical sector.
These findings underscore the importance of understanding the specific nature of bioethical commitments within each BRICS nation. Bioethical considerations are integral to the pharmaceutical industry, influencing trust, stakeholder relationships, and adherence to global ethical standards set by the World Health Organization. The alignment with WHO guidelines in the vision and mission statements of these companies reflects their commitment to ethical practices, patient welfare, and the advancement of healthcare.
The implications of this research are multifaceted. Firstly, it highlights opportunities for collaboration and knowledge sharing among BRICS countries to promote best practices in healthcare and pharmaceutical ethics. Secondly, it underscores the significance of ethical practices for building trust with stakeholders, including patients and investors. Thirdly, it emphasizes the role of national regulatory environments in influencing global bioethical standards.
The highlighted BRICS institutions, encompassing the Center for Bioethics and Culture in Brazil, the Institute of Philosophy in Russia, the Forum for Medical Ethics Society in India, the Center for Bioethics in China, and the Steve Biko Centre for Bioethics in South Africa, stand as vital pillars in shaping the ethical discourse within their respective nations. Their diverse roles, from fostering interdisciplinary research to promoting ethical practices, underscore their significance in addressing bioethical challenges. By embracing collaborative initiatives, tailoring training programs, engaging in policy advocacy, and championing transparency, these institutions can collectively propel a more ethically sound pharmaceutical industry across the BRICS nations.
Future research can delve into more in-depth company analysis, conduct comparative studies to identify influential factors in bioethical commitments, focus on policy development to enhance ethical practices, assess the long-term impacts of bioethical considerations, and investigate the effectiveness of ethical education and training for healthcare professionals. The research contributes to the promotion of public health, the alignment with global bioethics standards, and the enhancement of ethical practices in the pharmaceutical industry, ultimately benefiting society and patients. This study lays the foundation for a more comprehensive understanding of the pharmaceutical industry’s ethical practices, with potential implications for public health and global bioethics standards.
Conflict of interest: The authors of this study declare that there is no any conflict of interest.
References
- Tupara H. Ethics and health research: Decision making in Aotearoa New Zealand. AJOB Primary Research. 2012; 3(4): 40-52.
- Hummel P, Littler K, Reis A. Health Ethics & Governance at WHO: The importance of the Global Summit of National Ethics Committees. of Global Bioethics. 2023; 211.
- Frimpong-Mansoh Y. Intercultural global bioethics. Journal of Medical Ethics. 2023; 49(5): 339-340.
- Kirigia JM, Wambebe C, Baba-Moussa A. Status of national research bioethics committees in the WHO African region. BMC Medical Ethics. 2005; 6(1): 1-7.
- Hurst DJ. A Bioethics Tool for the Implementation of Global Principles by the Pharmaceutical Industry Duquesne University. 2017.
- Ezziane Z. Essential drugs production in Brazil, Russia, India, China and South Africa (BRICS): Opportunities and challenges. International journal of health policy and management. 2014; 3(7): 365.
- Zhang H, Zang Z. A bioethical examination of the use of human emotions as a film marketing tool on short video platforms: Normative morality applicable to film marketing matrix. Acta Bioethica. 2022; 28(2): 301-309.
- Vears DF, Gillam L. Inductive content analysis: A guide for beginning qualitative researchers. Focus on Health Professional Education: A Multi-disciplinary Journal. 2022; 23(1): 111-127.
- Ng NY, Ruger JP. Global health governance at a crossroads. Global health governance: The scholarly journal for the new health security paradigm. 2011; 3(2): 1.
- Organization WH. WHO framework convention on tobacco control: Guidelines for implementation of article 5. 3, Articles 8 To 14. World Health Organization. 2013.
- Organization WH. WHO guidelines on ethical issues in public health surveillance. 2017.
- Willison DJ, Ondrusek N, Dawson A, Emerson C, Ferris L. et al. What makes public health studies ethical? Dissolving the boundary between research and practice. BMC Medical Ethics. 2014; 15: 1-6.
- Graham J, Plumptre TW, Amos B. Principles for good governance in the 21st century (Vol. 15). Institute on governance Ottawa. 2003.
- German RR, Horan JM, Lee LM, Milstein B, Pertowski CA. Updated guidelines for evaluating public health surveillance systems; Recommendations from the Guidelines Working Group. 2001.
- Brock DW, Wikler D. Ethical challenges in long-term funding for HIV/AIDS. Health Affairs. 2009; 28(6): 1666-1676.
- Kickbusch I. Report of the Ebola interim assessment panel. 2015.
- Organization WH. WHO framework convention on tobacco control. Geneva: World Health Organization; 2003. 2019.
- Klaucke DN, Buehler JW, Thacker SB, Parrish RG, Trowbridge FL. Guidelines for evaluating surveillance systems. 1988.
- Tindana PO, Singh JA, Tracy CS, Upshur REG, Daar AS, et al. Grand challenges in global health: Community engagement in research in developing countries. Plos medicine. 2007; 4(9): e273.
- MacQueen KM, McLellan E, Metzger DS, Kegeles S, Strauss R. et al. What is community? An evidence-based definition for participatory public health. American journal of public health. 2001; 91(12): 1929-1938.
- Zakus JDL, Lysack CL. Revisiting community participation. Health policy and planning. 1998; 13(1): 1-12.
- H Barrett, DW Ortmann, L Dawson, A Saenz, C Reis, et al. Public health ethics: Cases spanning the globe. 2016.
- Scott JC. Seeing like a state: How certain schemes to improve the human condition have failed. yale university Press. 2020.
- Monitoring H. Impact using population-based surveys. Geneva: Joint United Nations Programme on HIV. AIDS. 2015.
- UNAIDS/PEPFAR. Considerations and guidance for countries adopting national health identifiers. In: Geneva Switzerland. 2014.
- Rose G. Sick individuals and sick populations Int J Epidemiol. 1985; 14(1): 32-38.
- Upshur RE, Morin B, Goel V. The privacy paradox: Laying Orwell’s ghost to rest. CMAJ. 2001; 165(3): 307-309.
- Bernstein AB, Sweeney MH, Control CfD. and Prevention. Public health surveillance data: Legal, policy, ethical, regulatory, and practical issues. MMWR Surveill Summ. 2012; 61: 30-34.
- Langat P, Pisartchik D, Silva D, Bernard C, Olsen K, et al. Is there a duty to share? Ethics of sharing research data in the context of public health emergencies. Public Health Ethics. 2011; 4-11.
- Frakt AB, Bagley N. Protection or harm? Suppressing substanceuse data. The New England journal of medicine. 2015; 372(20): 1879-1881.
- Sidel VW, Cohen HW, Gould RM. Good intentions and the road to bioterrorism preparedness. American journal of public health. 2001; 91(5): 716.