Research ArticleOpen Access, Volume 2 Issue 2
Effect of Health Education on the Knowledge of Pregnant Women on Iron and Folic Acid Supplements: A Stepped Wedge Cluster Randomized Trial
Haron Njiru1*; Mary W Gitahi1; Eunice Njogu2
1Department of Family Medicine, Community Health, and Epidemiology, Kenyatta University, Kenya.
2Department of Food, Nutrition and Dietetics, Kenyatta University, Kenya.
*Corresponding author: Haron Njiru
Department of Family Medicine, Community Health, and Epidemiology, Kenyatta University, Kenya.
Received : Mar 25, 2024 Accepted : Apr 15, 2024 Published : Apr 22, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Njiru Z © All rights are reserved
Citation: Njiru H, Gitahi MW, Njogu E. Effect of Health Education on the Knowledge of Pregnant Women on Iron and Folic Acid Supplements: A Stepped Wedge Cluster Randomized Trial. Epidemiol Public Health. 2024; 2(2): 1042.
Abstract
Iron deficiency poses a significant public health challenge during pregnancy. However, optimal uptake of antenatal micronutrients is hampered by lack of knowledge. We investigated the effect of health education on knowledge of women regarding antenatal Iron and Folic Acid Supplements (IFAS). In a 9-month trial, antenatal care clinics received a maternal IFAS awareness package, which included education for both health workers and pregnant women and health Information Education and Communication (IEC) materials. The study found that IFAS knowledge improved from 44.8% to 81.1%, a 36-percentage point increase. To enhance IFAS knowledge, hence uptake, the study recommends targeted health education emphasizing IFAS benefits, management of IFAS side effects, along with providing standardized information materials to the clinics.
Introduction
Micronutrients are necessary for growth, development, and normal functioning. Although required in minute amounts, their deficiency is a global public health challenge. The daily recommended dietary allowance for iron and folate increases by 50% during pregnancy from 18 milligrams (mg) to 27 mg, and from 400 micrograms (μg) to 600 μg for iron and folate respectively [1] due to the rapid multiplication of placental and fetal tissues. Diet alone cannot fully satisfy the increased demand, hence the need for daily micronutrient supplementation to avert deficiencies.
Deficiency of iron and folic acid during pregnancy increases the risk of anemia which is a leading cause of maternal deaths and adverse pregnancy outcomes. The global prevalence of Anemia in Pregnancy (AiP) is estimated at 38.2% or 32.4 million pregnant, making anemia the most common medical disorder in pregnancy. At 22% the Western Pacific region, the Americas, and the European region have the lowest prevalence of AiP [2] while lower income countries in South East Asia and Sub Saharan Africa have more than double the AiP burden at 46.2% [3]. The AiP prevalence is estimated at 36% in East Africa [4] and 62% in Kenya [5] with women in rural areas being more affected than those in urban areas at 50.8% and 29.5% respectively [6].
Studies have associated AiP with low-birth-weight babies and preterm births [7], susceptibility to childhood diarrhea and respiratory infections, and poor neurological development [8], increased perinatal, postnatal and under-5 mortality [9], and disrupted growth in adolescent mothers [10]. Indeed, pregnant women with severe AiP are twice as likely to die [11]. In Kenya, 10% and 20% of maternal and prenatal deaths respectively are attributable to anemia [12]. AiP can also trigger geophagy in pregnancy, a widespread practice detrimental to maternal and child health [13].
In populations at risk of iron deficiency, the World Health Organization (WHO) recommends a daily intake of 60 mg iron and 400 μg of folic acid as a standard of care for preventing AiP [1]. Antenatal iron supplements can reduce the risk of iron deficiency, halve the risk of neonatal death and reduce the incidence of low-birth-weight babies [14-16] while folic acid reduces the risk of underweight births, pre-eclampsia, placental abruption, preterm births, small for gestational age infants, and birth defects, and improve academic performance [17,18]. This sustains the gains made on the 1st, 4th and 5th Millennium Development Goals, contributes towards the 2nd Sustainable Development Goal’s (SDG) target of ending hunger and all forms of malnutrition by 2030 [19], and the first 3 (of the six) 2025 global nutrition targets [20].
Advocacy for Iron and Folic Acid Supplements (IFAS) as a strategy for anemia prevention is articulated in key global and national commitments and policy frameworks. The global nutrition target for 2025 is to achieve a 50% reduction of anemia among women of reproductive age. Compared to the 2011 baseline [4,20]. To meet this target, 18% of all the investments towards anemia should be directed towards antenatal IFAS [21]. The national IFAS program in Kenya is guided by various national policies on nutrition, food security and micronutrient deficiency control [22]. Kenya has also endorsed the Scaling up Nutrition (SUN) movement[23], which promotes antenatal IFAS as a core high impact intervention.
Despite the manifold benefits and the global advocacy, progress in the uptake of antenatal IFAS has been slow. This has been attributed to inadequate knowledge of the relationship between IFAS and anemia, lack of awareness on the risk of AiP and limited knowledge on the management of IFAS side effects [12,24-27].
Health education and promotion play crucial roles in enhancing knowledge, shaping beliefs, and influencing attitudes. Consequently, they contribute to greater utilization of health services [28,29]. Consistent health education fosters deeper understanding and encourages positive behavioral shifts [24]. Health education and promotion should strategically target the health workers and pregnant women. Health workers play a pivotal role in enhancing health literacy, making their empowerment essential for successful implementation of facility-based interventions, [30,31] equally essential is the need to actively engage the pregnant women. The behavior change interventions should create awareness about a desired behavior, motivate change by underscoring the positive and negative consequences, and provide opportunities for practicing the desired behaviors [32].
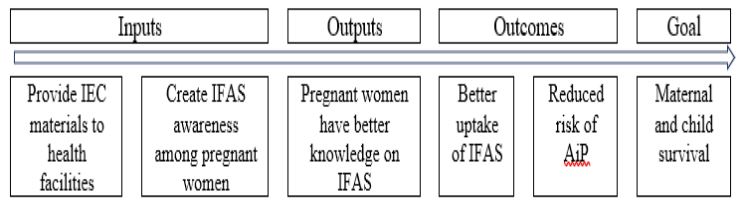
The MIA trial sought to enhance knowledge, modify attitudes and beliefs, and skills to positively influence IFAS uptake using multiple behavior change techniques [28,29]. This was achieved through face-to-face health education sessions provided to pregnant women by health workers, provision of study IEC materials and the pill reminder cards. The trial’s chain of results is depicted in Figure 1.
Methodology
Study design
A stepped wedge Cluster Randomized Trial (swCRT) was designed using Antenatal Care (ANC) clinics as units of randomization. This design was suitable since individual randomization was impractical for logistical and ethical reasons. All clusters started the trial at the same time and acted as controls until they were randomized to crossover from control to the intervention phase.
Study setting
The Maternal IFAS Awareness (MIA) trial was conducted in Embu County, Kenya. With an estimated population of 609,000, Embu county is the 12th most populous County, out of the 47 counties in Kenya [33]. About half (43%) of the pregnant women in the County attend ANC, 6% of them consume IFAS for at least 90 days [34], just over a half (53%) do not complete ANC visits and one in every six are anemic [35]. The study population were the pregnant women attending ANC clinics at the selected public health facilities that offered antenatal care.
Intervention
The study intervention was grounded on the social cognitive theory of behavior change [32]. The intervention entailed (1) IFAS information sessions with ANC service providers delivered in 60 minutes lunchtime sessions to minimize interruption of service delivery, (2) Daily IFAS literacy sessions with pregnant women, and (3) provision of Information, Education and Communication (IEC) materials (Pill Reminder Card (PRC) and MIA wall calendars) to pregnant women. The calendars had IFAS messages and were pre-populated with personalized ANC clinic return dates. All IEC materials were adapted from the national IFAS program and customized to fit the local context based on evidence from the baseline facility assessment.
The MIA trial ran for 9 months (June 2022 to February 2023). This entailed one month for baseline data collection and customization of health education messages, seven months intervention, and another month to finalize the data collection and the handover processes. Execution of the study including availability of supplies and continuity of counselling was enhanced through biweekly spot-checks and monthly audits. The intervention is described in detail in the study protocol [36].
Sample size determination
The number of clusters to enroll in MIA trial was estimated using the two equations proposed by Hayes & Bennett [37]. A total of 12 clusters were required for the survey. With a 5% margin of error, assuming 6% IFAS uptake and a 5% non-response rate, the minimum number of respondents for baseline and endline surveys was 92 women Detailed sample size calculation and the sampling technique is shown in the protocol [36].
Data collection and analysis
Data was collected using an interviewer-administered questionnaire and pill counting. The questionnaire obtained information on sociodemographic characteristics and levels of IFAS knowledge among women across 5 areas: Benefits of IFAS, gestation age at which IFAS should be initiated, best time to take IFAS, daily dose requirements, and management of IFAS side effects.
Proportions were used to describe the demographic characteristics and sources of IFAS information. Additionally, the t-test statistic was employed to compare changes in knowledge levels between control and intervention phases. The precision of estimates was based on p-values and 95% Confidence Intervals (CI), with significance set at 0.005.
Logistical and ethical considerations
The trial was registered in the Pan African Clinical Trial Registry (PACTR202202775997127), permit obtained from National Commission for Science, Technology and Innovation (NACOSTI/P/22/16168), and clearance sought from the County health authorities. Ethical approval was granted by Kenyatta University Ethics Review Committee (PKU/2443/11575).
Findings
A total of 11,569 ANC visits were registered at the 12 clusters between June 2022 and January 2023. All women receiving ANC services during the intervention phase were provided with PRCs to monitor their IFAS uptake. A total of 192 pregnant women (96 at baseline and 96 during the intervention period) participated in the exit surveys. The average age of study participants was 25 years, most were married (78%), unemployed (64%), lived in rural areas (66%), nulliparous (60%), and had started ANC at 16 weeks (Table 1).
Women’s knowledge on IFAS was assessed at baseline and during the intervention. The knowledge score improved from 44.8% (95% CI: 38.80-50.71) at baseline to 81.1% (95% CI: 79.75-82.49) during the intervention. This reflected a 36.36 (95% CI: 31.82-40.91) percentage point improvement in levels of IFAS knowledge.
At baseline, knowledge on the daily dose requirement was the highest at 95.5% while knowledge on of IFAS side effects was the lowest at 32.6%, still the latter tailed following the intervention at 72.5%. Knowledge on the best time to take IFAS showed the greatest improvement at 47.7 percentage points, while knowledge on the benefits of IFAS was the least impacted by the intervention with an effect estimate of 34.7 percentage points (Table 2).
The main source of information for IFAS during the intervention was from conversations with skilled health workers and peers. This was a shift compared with the baseline situation when the main sources of IFAS information had been community health workers (Table 3).
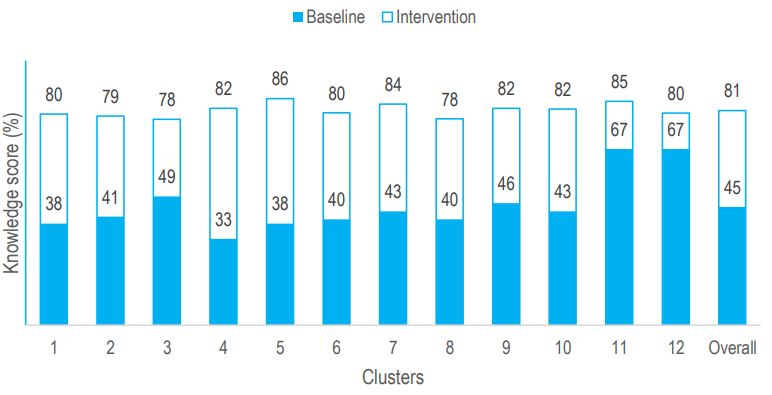
Improvements in levels of IFAS knowledge following the intervention were observed across all clusters (Figure 2). Furthermore, a positive but statistically insignificant correlation was observed between knowledge and number of ANC contacts. For every additional ANC contact, knowledge levels improved by a coefficient of 1.1 (95% CI: -2.1-4.3).
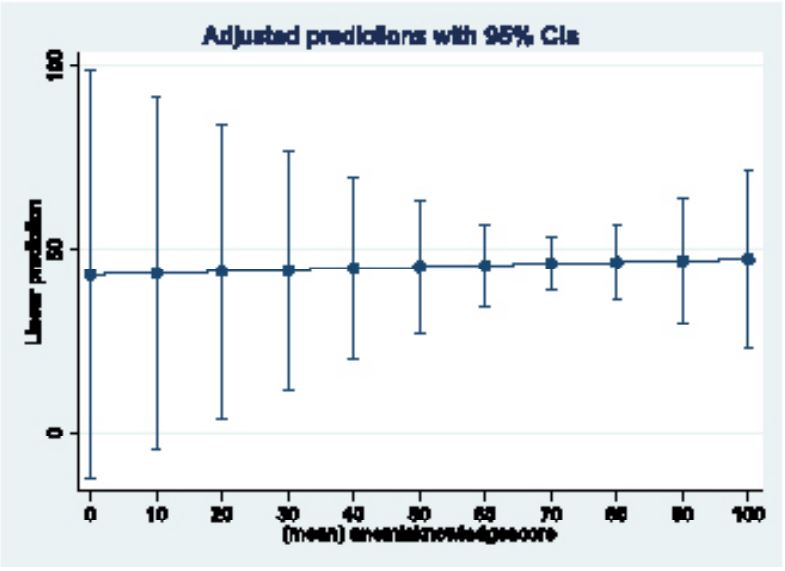
Pearson correlation coefficient (r) was calculated to evaluate the relationship between IFAS knowledge and uptake. Although a positive correlation was observed, it was weak (r=0.228, indicating only a 5% variability between knowledge and uptake). An adjusted linear prediction model revealed that even with a perfect 100% knowledge score, IFAS uptake would only reach a maximum of 47.3% (Figure 3).
Table 1: Demographic characteristics of survey respondents at baseline.
| Characteristic | Respondents (n=96) |
|---|---|
| Age (years) | 25.3(5.9) |
| 20 years and above | 82(85%) |
| Below 20 years | 14(15%) |
| Marital status | |
| Married | 75(78%) |
| Not married | 21(22%) |
| Level of education | |
| Primary | 38(40%) |
| Secondary | 43(45%) |
| College | 15(16%) |
| Place of residence | |
| Rural | 63(66%) |
| Urban | 33(34%) |
| Employment status | |
| Employed/business | 35(36%) |
| Unemployed | 61(64%) |
| Parity | |
| Multiparous | 38(40%) |
| Nulliparous | 58(60%) |
| Gestation at first ANC | 16.2(8.1) |
| Within initial 12 weeks | 36(38%) |
| After 12 weeks | 60(62%) |
| Data are n (%) or mean (SD) | |
Table 2: Knowledge on IFAS.
| Aspect of IFAS knowledge | Control | Intervention | Effect estimate |
|---|---|---|---|
| mean (SD) | mean (SD) | (95% CI) | |
| Benefits of IFAS | 47.7(26.3) | 82.4(15.2) | 34.7(27.5-41.8) |
| Gestation age to start IFAS | 56.8(52.1) | 98.8(16.7) | 42.0(31.0-53.0) |
| Best time to take IFAS | 43.1(58.1) | 90.9(28.9) | 47.7(34.1-61.3) |
| Daily dose of IFAS | 95.5(21.1) | 100.0(--) | 4.5(0.1-9.0) |
| Management of side effects | 32.6(22.7) | 72.5(8.0) | 40.0(34.6-45.3) |
| Overall | 44.8(19.6) | 81.1(6.5) | 36.4(31.8-40.9) |
Table 3: Sources of IFAS information.
| Source of IFAS information | Control (n=44) |
Intervention (n=88) |
Pearson’s x2 p-value |
|---|---|---|---|
| Skilled health workers | 21(48%) | 74(84%) | <0.001* |
| Peers / friends | 21(48%) | 70(80%) | <0.001* |
| Community health workers | 26(59%) | 46(52%) | 0.5 |
| Family member | 20(45%) | 43(49%) | 0.7 |
| Magazine / newspapers / posers | 24(55%) | 41(47%) | 0.4 |
| Radio/ TV | 11(25%) | 20(23%) | 0.8 |
| Other sources | 10(23%) | 17(19%) | 0.6 |
| Facebook/ Internet | 5(11%) | 12(14%) | 0.7 |
Discussion
The baseline levels of IFAS knowledge among pregnant women were low, at 44.8%. Knowledge on management of side effects and IFAS benefits were the lowest at 32% and 47% respectively. Only daily dose had a knowledge score above 90% at baseline. Low levels of IFAS knowledge have been shown in other studies. A quasi experimental study in Kiambu - Kenya showed a 57% IFAS knowledge at baseline [38] and a general lack of awareness in western Kenya and Ethiopia [27,39].
Limited knowledge on benefits of any intervention coupled with limited knowledge on how to manage potential side effects, as observed in the MIA trial at baseline, could hinder uptake, especially if the intervention has side effects, and when the benefit of an intervention is not instant, as is the case with IFAS.
The IFAS knowledge increased from 44.8% at baseline to 81.1% during the intervention, a difference of 36.4 percentage points. The baseline assessment had identified key knowledge gaps and the design of IEC materials for the MIA trial had been customized to address these gaps among health workers and pregnant women. In addition, the IFAS sessions with health workers at the study inception together with the availability of relevant IEC materials provided an enabling environment for discussion between health workers and pregnant women [31] which could explain the observed improvement in knowledge levels. Furthermore, the main sources of information switched from community health workers at baseline to skilled health workers and peers during the intervention period. This could imply that in addition to boosting their confidence, [31] providing health workers with IEC materials provided them with a structured and better way to counsel women on IFAS, which in turn increased the women’s level of knowledge on antenatal IFAS.
That participants in the MIA trial had significantly higher knowledge levels after the intervention is promissory. However, not achieving 100% knowledge levels is disappointing. This failure could have been due to varying knowledge-retention capacities among respondents owing to the differences in levels of academic achievements, and the fidelity of the intervention implementation. Faced with a heavy workload occasioned by the free maternity services policy, health workers are likely to pay less emphasis on the needs of individual women, as required for the MIA intervention, and this could have weakened the intensity of knowledge transfer. This phenomenon is reinforced with the observation that knowledge improved with increasing number of ANC contacts, albeit insignificantly. A similar shortfall was observed in a study in Uganda where IFAS knowledge improved from 57 to 92% [38].
There was a positive but weak correlation between the levels of IFAS knowledge and uptake. That better knowledge leads to improved uptake of IFAS has also been reported in other studies [26,38,40,41]. Those who had better knowledge were more likely to adhere but only up to a maximum of 47.3%. This implies that while knowledge is necessary for uptake, knowledge alone is not sufficient to achieve sustained uptake. The observation that coverage exceeded 47.3% indicates presence of other factors that motivated women to take IFAS over and above their knowledge about IFAS. This could be the effect of PRC, but needs further exploration.
Having a PRC with information on IFAS served multiple functions: First, it was a mnemonic, and secondly it was informational. On the other hand, wall calendars served as visual reminders of the importance of IFAS, additionally reminding women about the ANC return date during which their IFAS supplies would be refilled, they would also interact with peers, and learn more about IFAS from the healthcare providers. Furthermore, health workers were also provided with IEC materials and a structured way of communicating about IFAS to women, with a focus on importance of IFAS, potential side effects, and how to mitigate the latter. The importance of visual aids such as the MIA wall calendars and PRC have been previously documented, though not in relation to antenatal IFAS [42].
Conclusion
Pregnant women have low levels of IFAS knowledge. Women are generally oblivious of the IFAS benefits. This hinders optimal uptake of IFAS, a situation exasperated by limited knowledge of women on how to manage the IFAS side effects. The levels of IFAS knowledge increased with the number of ANC contacts and that IFAS knowledge had a positive, albeit weak, influence on IFAS uptake.
Recommendations
The MIA trial has shown that public health education has the potential to improve IFAS knowledge and thereby uptake in antenatal care settings. The government should provide ANC clinics with IFAS guidelines and IEC materials to ensure pregnant women receive comprehensive IFAS information as part of routine ANC care. Furthermore, health workers should be sensitized about the MoH IFAS guidelines to improve the quality and consistency of messages passed to pregnant women, and lastly, health workers should educate pregnant women on IFAS at every ANC visit to improve their IFAS knowledge and uptake.
Declarations
Author contributions: HN drafted the study findings, interpretation of the findings and discussion. All authors contributed to refinement of the findings and approved the final manuscript.
Funding: This research received no grant from any funding agency.
Competing interests: The authors have no financial or other competing interests to declare.
Acknowledgments: We extend our heartfelt gratitude to the Kenya Ministry of Health, particularly the Embu County health management team and the dedicated facility in-charges. Their collaboration was instrumental in the success of this trial. We also express our appreciation to every expectant woman whose participation was the cornerstone of this trial, and to our research assistants, Caleb Karanja and Rose Kendagor.
References
- WHO. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization. 2016.
- WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization. Geneva: World Health Organization. 2015.
- WHO. Prevalence of anaemia in pregnant women (aged 15-49) (%). Global Health Observatory: WHO. 2019.
- Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. The Lancet Global Health. 2013; 1(1): e16-e25. doi: 10.1016/s2214-109x(13)70001-9.
- Harika R, Faber M, Samuel F, et al. Micronutrient Status and Dietary Intake of Iron, Vitamin A, Iodine, Folate and Zinc in Women of Reproductive Age and Pregnant Women in Ethiopia, Kenya, Nigeria and South Africa: A Systematic Review of Data from 2005 to 2015. Nutrients. 2017; 9(10). doi: 10.3390/nu9101096.
- MoH. Kenya National Micronutrient Survey 2011. 2011.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middleincome countries: systematic review and meta-analysis. Am J Clin Nutr. 2016; 103(2): 495-504. doi: 10.3945/ajcn.115.107896.
- Christian P, Kim J, Mehra S, et al. Effects of prenatal multiple micronutrient supplementation on growth and cognition through 2 y of age in rural Bangladesh: the JiVitA-3 Trial. Am J Clin Nutr. 2016; 104(4): 1175-1182. doi: 10.3945/ajcn.116.135178.
- Abir T, Ogbo FA, Stevens GJ, et al. The impact of antenatal care, iron-folic acid supplementation and tetanus toxoid vaccination during pregnancy on child mortality in Bangladesh. PLoS One. 2017; 12(11): e0187090. doi: 10.1371/journal.pone.0187090.
- WHO. Guideline: Implementing effective actions for improving adolescent nutrition. Geneva. 2018: 33-37.
- Daru J, Zamora J, Fernandez-Felix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health. 2018; 6(5): e548-e554. doi: S2214-109X(18)30078-0.
- Kamau MW, Mirie W, Kimani S. Compliance with Iron and Folic Acid Supplementation (IFAS) and associated factors among pregnant women: Results from a cross-sectional study in Kiambu County, Kenya. BMC Public Health. 2018; 18(1): 580. doi: 10.1186/s12889-018-5437-2.
- Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: A literature review. Obstet Gynecol Surv. 2011; 66(7): 452-459. doi: 10.1097/OGX.0b013e318232a034.
- Pena-Rosas JP, De-Regil LM, Dowswell T, et al. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2012; 12: CD004736. doi: 10.1002/14651858.CD004736.pub4.
- Titaley CR, Dibley MJ. Antenatal iron/folic acid supplements, but not postnatal care, prevents neonatal deaths in Indonesia: Analysis of Indonesia Demographic and Health Surveys 2002/2003–2007 (a retrospective cohort study). BMJ Open. 2012; 2(6). doi: 10.1136/bmjopen-2012-001399.
- Imdad A, Bhutta ZA. Routine iron/folate supplementation during pregnancy: Effect on maternal anaemia and birth outcomes. Paediatr Perinat Epidemiol. 2012; 26(Suppl 1): 168-177. doi: 10.1111/j.1365-3016.2012.01312.x.
- Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015; 66(Suppl 2): 22-33. doi: 10.1159/000371618.
- Kim MW, Ahn KH, Ryu KJ, et al. Preventive Effects of Folic Acid Supplementation on Adverse Maternal and Fetal Outcomes. PLoS One. 2014; 9(5): e97273. doi: 10.1371/journal.pone.0097273.
- Grosso G, Mateo A, Rangelov N, et al. Nutrition in the context of the Sustainable Development Goals. Eur J Public Health. 2020; 30(Suppl_1): i19-i23. doi: 10.1093/eurpub/ckaa034.
- WHO. Global nutrition targets 2025: Policy brief series (WHO/NMH/NHD/14.2). Geneva: World Health Organization. In: WHO, ed. 2014.
- Walters D KJ, Eberwein JD, Shekar M. An investment framework for meeting the global nutrition target for anemia. Washington DC: World Bank. 2017.
- MoH. Kenya National Iron and Folic Acid Supplementation (IFAS) Communication Strategy (2013-2017). 2013.
- Scaling Up Nutrition. 2023. www.scalingupnutrition.org.
- Siekmans K, Roche M, Kung’u Jacqueline K, et al. Barriers and enablers for Iron Folic Acid (IFA) supplementation in pregnant women. Maternal & Child Nutrition. 2017; 12532: e12532. doi: 10.1111/mcn.12532.
- Billah SM, Raynes-Greenow C, Ali NB, et al. Iron and Folic Acid Supplementation in Pregnancy: Findings from the Baseline Assessment of a Maternal Nutrition Service Programme in Bangladesh. 2022; 14(15): 3114.
- Gonzalez-Casanova I, Nguyen PH, Young MF, et al. Predictors of adherence to micronutrient supplementation before and during pregnancy in Vietnam. BMC Public Health. 2017; 17(1): 452. doi: 10.1186/s12889-017-4379-4.
- Martin SL, Seim GL, Wawire S, et al. Translating formative research findings into a behaviour change strategy to promote antenatal calcium and iron and folic acid supplementation in western Kenya. Matern Child Nutr. 2017; 13(1). doi: 10.1111/mcn.12233.
- Briscoe C, Aboud F. Behaviour change communication targeting four health behaviours in developing countries: A review of change techniques. Soc Sci Med. 2012; 75(4): 612-621. doi: 10.1016/j.socscimed.2012.03.016.
- Van Achterberg T, Huisman-de Waal GG, Ketelaar NA, et al. How to promote healthy behaviours in patients? An overview of evidence for behaviour change techniques. Health Promot Int. 2011; 26(2): 148-162. doi: 10.1093/heapro/daq050.
- Presseau J, Mutsaers B, Al-Jaishi AA, et al. Barriers and facilitators to healthcare professional behaviour change in clinical trials using the Theoretical Domains Framework: A case study of a trial of individualized temperature-reduced haemodialysis. Trials. 2017; 18(1): 227. doi: 10.1186/s13063-017-1965-9.
- Giguere A, Zomahoun HTV, Carmichael PH, et al. Printed educational materials: Effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2020; 8: CD004398. doi: 10.1002/14651858.CD004398.pub4.
- Bandura A. Social cognitive theory: An agentic perspective. Annual review of psychology. 2001; 52: 1-26. doi: 10.1146/annurev.psych.52.1.1.
- KNBS. 2019 Kenya Population and Housing Census. Distribution of Population by Administrative Units. 2019.
- KNBS. Kenya Demographic and Health Survey 2014. Rockville, Maryland, USA: Kenya National Bureau of Statistics (KNBS) and ICF Macro. 2015.
- MoH. Kenya District Health Information System. ed. 2021.
- Njiru H, Njogu E, Gitahi MW, et al. Effectiveness of public health education on the uptake of iron and folic acid supplements among pregnant women: A stepped wedge cluster randomised trial. BMJ Open. 2022; 12(9): e063615. doi: 10.1136/bmjopen-2022-063615.
- Hayes RJ, Bennett S. Simple sample size calculation for clusterrandomized trials. Int J Epidemiol. 1999; 28(2): 319-326.
- Kamau M, Mirie W, Kimani S, et al. Effect of community based health education on knowledge and attitude towards iron and folic acid supplementation among pregnant women in Kiambu County, Kenya: A quasi experimental study. PLoS One. 2019; 14(11): e0224361. doi: 10.1371/journal.pone.0224361.
- Wana EW. Predictors of prenatal iron folic acid supplement utilization in Wolaita, South Ethiopia: A community based crosssectional study (quantitative and qualitative approach). BMC Pregnancy Childbirth. 2020; 20(1): 243. doi: 10.1186/s12884-020-02883-2.
- Mabuza GN, Waits A, Nkoka O, et al. Prevalence of iron and folic acid supplements consumption and associated factors among pregnant women in Eswatini: A multicenter cross-sectional study. BMC Pregnancy and Childbirth. 2021; 21(1): 469. doi: 10.1186/s12884-021-03881-8.
- Saragih ID, Dimog EF, Saragih IS, et al. Adherence to Iron and Folic Acid Supplementation (IFAS) intake among pregnant women: A systematic review meta-analysis. Midwifery. 2022; 104: 103185. doi: 10.1016/j.midw.2021.103185.
- Lillevoll KR, Vangberg HC, Griffiths KM, et al. Uptake and adherence of a self-directed internet-based mental health intervention with tailored e-mail reminders in senior high schools in Norway. BMC Psychiatry. 2014; 14: 14. doi: 10.1186/1471-244X14-14.