Short CommentaryOpen Access, Volume 2 Issue 2
ESKAPE Monitoring in Bronchoalveolar Lavage of ICU Patients with CNS Pathologies
Olga S Polyakushkina1; Anna V Pakhilova-Popova2; Raisa T Tairova3; Olga I Patsap4*
1Department of Clinical Epidemiology and Prevention, Clinical Epidemiologist, Federal Center of Brain Research and Neurotechnologies of FMBA of Russia, Moscow, Russia.
2Clinical Microbiologist, Clinical Diagnostic Laboratory, Federal Center of Brain Research and Neurotechnologies of FMBA of Russia, Moscow, Russia.
3Chief Medical Officer, Federal Center of Brain Research and Neurotechnologies of FMBA of Russia, Moscow, Russia.
4Head of Pathology Department, Federal Center of Brain Research and Neurotechnologies of FMBA of Russia, Moscow, Russia.
*Corresponding author: Olga I Patsap
Head of Pathology Department, Federal Center of Brain Research and Neurotechnologies of FMBA of Russia, Moscow, Russia.
Email: olga@patsap.ru
Received : Mar 09, 2024 Accepted : Apr 04, 2024 Published : Apr 11, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Patsap OI © All rights are reserved
Citation: Polyakushkina OS, Pakhilova-Popova AV, Tairova RT, Patsap OI. ESKAPE Monitoring in Bron choalveolar Lavage of ICU Patients with CNS Pathologies. Epidemiol Public Health. 2024; 2(2): 1039.
Abstract
Keywords: Healthcare-associated infections; ESKAPE monitoring; WHONET.
Introduction
Healthcare-Associated Infections (HAIs) have been an issue for decades [1]. Increasing microbial resistance to the antibiotics used for treatment of Ventilator-Associated Pneumonia (VAP) creates certain difficulties for clinicians in treating this pathology and increases the risk of lethal outcome, entailing economic losses for clinics and fostering the antibiotic resistance of the pathogens circulating in an ICU. The risks are associated with many invasive procedures; weakened immune status; decreased efficiency of barrier functions of respiratory-tract mucous membranes; impaired nutritional status (decreased bloodprotein values), etc [2].
Results and discussion
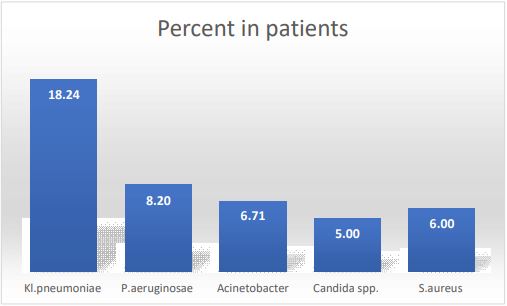
Between 2021 and 2023 at the Federal Center of Brain Research and Neurotechnologies of Federal Medical Biological Agency, the pathogens detected in ICU patients were investigated to establish an optimal protocol for empirical antibiotic VAP therapy. A total of 1074 Bronchoalveolar-Lavage (BAL) samples from 625 patients were examined to have identified the main culprits such as Kl. pneumoniae, P. aeruginosae, Acinetobacter, Candida spp. Kl. pneumoniae was isolated in 18.24% of patients on ventilator, P. aeruginosae in 8.2%, Acinetobacter in 6.71%, Candida spp. in 5%, and S. aureus in in 6% (Figure 1). The antibiotic sensitivity profiles of all isolated pathogens were investigated, their phenotypes and antibiotic resistance classes were established. The data obtained enabled the clinical pharmacologist to develop a protocol for effective empirical antibacterial therapy to be administered to a VAP patient before their BAL examination results became available. Regular (once every 6 months) examination for BAL pathogens allowed one to adjust the empirical antibiotic therapy, amend a purchase order for the pharmacy, and timely withdraw the antibiotics the pathogens isolated from BAL were no longer sensitive. Having withdrawn the “ineffective” antibiotics, we did not stop monitoring the pathogens’ sensitivity for them, which led to the so-called antibiotic renaissance when the drugs restored their efficacy in affecting the main gram-negative pathogens.
The information on microbiological tests performed and antibiotic agents identified was collected using the WHONET software recommended by the WHO for microbiological monitoring in healthcare facilities.
Conclusion
Analysis of obtained results has brought us to the conclusion that the main threat for the ICU patients on ventilator is gramnegative pathogens with Kl. pneumoniae being the main culprit in causing VAP. Rational use of the results of antibiotic sensitivity analysis enables one to start antibiotic therapy before the results of BAL examination become available; timely adjust the empirical antibiotic therapy, accounting for the isolated microflora; and withdraw the antibiotics that have exhausted their efficacy.
Declarations
Funding: The work was completed without funding.
Conflict of interest: Authors declare no conflict of interest.
Ethics approval: The work was approved by the local ethics committee of Federal Center of Brain Research and Neurotechnologies of FMBA of Russia, Moscow, Russia. Approval #07/19- 02-2024.
The work was performed without involving patients or laboratory animals.
References
- Centers for Disease Control and Prevention [https://www.cdc. gov/hai/index.html].
- Ivanov FV, Gumilevskii BY. Microbiological monitoring of healthcare-associated infections. International Research Journal. 2023; 12(138). doi: 10.23670/IRJ.2023.138.210.