Research ArticleOpen Access, Volume 2 Issue 1
Epidemiology of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis
Chaoping Li1 ; Yaoyu Ying2 ; Yang Zheng3 ; Xiang Li4,5; Lei Lan4,5*
1Kidney Disease Center, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
2Department of Medical Affairs, The Second Affiliated Hospital of Soochow University, Suzhou, China.
3Department of Allergy, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
4State Key Laboratory for the Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
5National Clinical Research Center for Infectious Diseases, Hangzhou, China.
*Corresponding author: Lei Lan
National Clinical Research Center for Infectious Diseases, Hangzhou, China.
Received : Jan 22, 2024 Accepted : Feb 16, 2024 Published : Feb 23, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Lan L © All rights are reserved
Citation: Li C, Ying Y, Zheng Y, Li X, Lan L. Epidemiology of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Epidemiol Public Health. 2024; 2(1): 1034.
Abstract
Background: Irritable Bowel Syndrome (IBS) is one of the most common functional bowel disorders, but its prevalence appears to vary widely between different countries. In this systematic review and meta-analysis, we aimed to provide an updated comprehensive estimate of IBS prevalence at the country, regional, and global levels.
Methods: The PubMed, EMBASE and Web of Science databases were searched (from January 1990 to December 2022), and eligible studies reporting the prevalence of IBS were selected. We extracted the prevalence data from the included studies, and the meta-analysis was completed using a random-effects model.
Results: In total, 131 publications reporting 133 records from 39 countries with 292,951 participants were identified for our analysis. The global pooled prevalence of IBS was 15.0% (95% CI: 13.4%-16.6%), and the pooled prevalence of IBS varied substantially between countries and regions. The highest pooled prevalence of IBS was 18.9% in South America, and the lowest was 11.0% in Southeast Asia. The pooled prevalence of IBS in females was 15.0%, while that in males was 11.0%. The pooled prevalence of IBS was 14.2% from 1990 to 2000, 11.4% from 2001 to 2010 and 16.5% from 2011 to 2022.
Conclusion: The present meta-analysis provides comprehensive and useful information on the epidemiology of IBS. The prevalence of IBS varies strikingly by country and region, even when the same diagnostic criteria were applied. Furthermore, future studies will be needed to assess the prevalence of IBS as the prolonged symptoms of COVID-19 infection.
Keywords: Irritable Bowel Syndrome (IBS); Prevalence; Risk factors; Meta-analysis.
Introduction
Irritable Bowel Syndrome (IBS) is a chronic functional bowel disorder characterized by abdominal pain or discomfort associated with a change in stool form or frequency [1,2]. Studies have shown that IBS affects 7% to 21% of the general population worldwide [3,4]. The prevalence of IBS varies by country and population. It has been reported that the global prevalence of IBS in adults is approximately 8.8%, 7.1% in North America and 12.6% in Asia [5,7]. Defined by the Rome IV criteria, it can be classified as constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), mixed stool pattern (IBS-M), and amorphous type (IBS-U) [8]. Studies have revealed that IBS is more common in females than in males in the Western world [9]. This may be related to female estrogen, which promotes the improvement of intestinal sensitivity or immune dysfunction [10,11]. While the causes of IBS are still not completely clear, it is believed that they are induced by psychosocial status, food intolerance, infection, and vitamin D deficiency [12,16]. The natural history of IBS is one of relapsing and remitting symptoms, which can negatively affect quality of life and work productivity and represent a considerable economic burden [8,17,19]. Thus, it is important to estimate the global prevalence of IBS to understand the distribution and burden of this disease.
In the absence of a diagnostic gold standard for IBS, the symptom-based diagnostic criteria were developed by consensus among experts in the field. These criteria to define IBS have evolved over the years, with the Rome III criteria in use since 2006 and the Rome IV criteria published in 2016. Thus, when using the different criteria, there may be a difference in the prevalence of IBS. Although the prevalence of IBS has been studied systematically, a few previous studies were using the historical definitions of IBS[5,20]. Only six studies reported prevalence according to the Rome IV criteria [21]. Concurrently, since COVID-19 pandemic in 2019, the Post-COVID-19 Syndrome (PCS) has raised serious concerns, as a complex and multifactorial condition that involved with the gastrointestinal symptoms, such as diarrhea, nausea, and abdominal pain. It was reported that that SARS-CoV-2 infection can result in the development of IBS that may be part of a systemic PCS [22,23].
Therefore, to understand the epidemiology of IBS more completely, we conducted a systematic review and meta-analysis to provide a comprehensive estimate of IBS prevalence and its changing trends between 1990 and 2022.
Methods
Search strategy: In this systematic review, we searched the PubMed, EMBASE and Web of Science databases for published literature between January 1990 and December 2022. The literature was searched by using a comprehensive set of search terms. Terms used in this search included a combination of the following terms and their corresponding synonyms: irritable bowel syndrome, IBS, irritable colon, spastic colon, functional adj5 bowel, combined with the set operator “OR”. There were no language restrictions. Further search strategies are detailed in Supplementary Table 1.
Data screening and extraction: To be eligible [21], studies had to recruit at least 50 participants and studies were limited to cross-sectional surveys that reported the prevalence of IBS. Two independent authors (LCP and YYY) screened all citations and identified the eligible studies. Any disagreements were resolved by discussion to reach consensus and, when necessary involved a third review author (LL). The authors (ZY,LX) developed a data extraction form of which parts were adapted from the Checklist for Prevalence. Study data were also extracted and cross-checked by two investigators independently. From each included study, the following data were extracted: publication year, the name of the first author, country of study population, study period, sample size, mean or median of participants’ age, the number of males and females, the number of participants with IBS, the number of males and females with IBS, the criteria used to define IBS, the method of data collection (interview, self-completed questionnaire and telephone) and the number of IBS subtypes (IBS-C, IBS-D, IBS-M, IBS-U and IBS-A). When data were missing or unsuitable for analysis, we contacted the authors to request further information.
Study quality assessment: The bias risk of the included studies was evaluated using the risk of bias tool developed specifically for prevalence studies [24]. Based on this scale, studies were assessed in terms of selection bias, nonresponse bias, measurement bias, and analysis bias. This scale consisted of 10 items, and each study was given a total risk score from 0 to 9. Studies with a score of 4 or more were classified as having a higher risk of bias. Risk of bias assessments were conducted independently by two reviewers (LCP and YYY), and any discrepancies in rating were resolved by a third reviewer (LL) through discussions and consensus. The results of the bias risk analysis are shown in Supplementary Table 2.
Statistical analysis: Heterogeneity between studies was estimated by using the I2 statistic, and the χ2 test with a P value was used to define a statistically significant degree of heterogeneity. Due to the expected high heterogeneity, data were pooled by using a random-effects model to give a more conservative estimate of the prevalence of IBS. The prevalence of IBS was compared according to the geographic region, country, publication year, gender, criteria used to define IBS and subtype of IBS (IBS-D, IBS-C, IBS-M, IBS-U) by using an odds ratio (OR) with a 95% Confidence Interval (CI). We conducted influential analyses by serially excluding each study to estimate the effect of individual studies on the overall prevalence estimates. The publication bias was assessed by applying the funnel plot and Egger’s test in the meta-analysis. All analyses were performed using R software (version 4.0.3). A P value of <0.05 was considered statistically significant.
Results
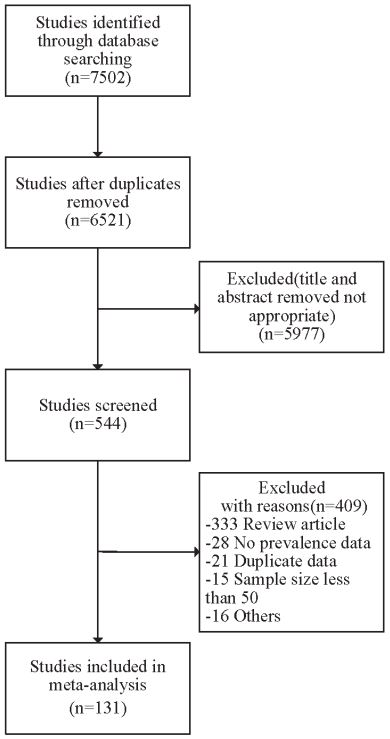
Study selection and characteristics: Of the 7,502 identified records, 981 duplicate citations were excluded. After screening the titles and abstracts, 544 potentially relevant articles were independently reviewed for full text. In total, 131 studies from 39 countries were included in the final meta-analysis. A flow chart of the study selection is presented in Figure 1. Detailed characteristics of all included studies are summarized in Supplementary Table 3. Forty-three studies were conducted in the Western Pacific region, twenty-nine studies in the European region, twenty-eight studies in the Eastern Mediterranean region, fifteen studies in North America, nine studies in Southeast Asia, eight studies in South America, and one study in the African region. Among the included studies, 292,951 participants were identified to calculate the global prevalence of IBS.
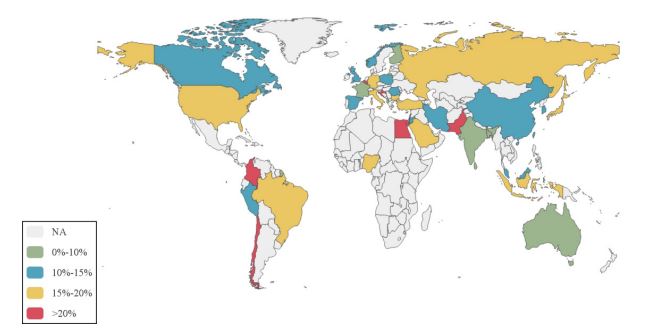
Global prevalence of IBS according to region and country: The pooled global prevalence of IBS conducted in this metaanalysis was 15.0% (95% CI: 13.4%-16.6%). By Egger’s test, there was less likely publication bias in this meta-analysis (P=0.22).The majority of studies were conducted in China and the USA. In all, the pooled prevalence of IBS ranged from 0% to 10% in 7 countries (LK, FI, FR, AL, IN, BD, AU), 10% to 15% in 12 countries (UK, IR, CA, MY, PL, NO, KR, CN, PE, ES, JO, RO), 15% to 20% in 12 countries (US, NG, DE, JP, IT, ID, MT, TR, SA, BR, RU, BG) and >20% in 8 countries (LB, CO, CL, HR, PS, BE, PK, EG). The highest and lowest prevalence of IBS occurred in Egypt (31.7%, 95% CI: 21.7%-36.4%) and Sri Lanka (5.0%, 95% CI: 4.1%-5.9%). The prevalence of IBS by country is shown in Figure 2. The pooled prevalence of IBS according to geographic location is provided in Table 1. The highest prevalence of IBS was in the South American region (18.9%, 95% CI, 95% CI: 15.3%-22.8%), followed by the Eastern Mediterranean (17.5%, 95% CI: 13.8%-21.5%), African (16.6%, 95% CI: 15.0%-18.3%), European (15.9%, 95% CI: 12.7%-19.3%), North American (14.5%, 95% CI: 9.0%-21.2%) and Western Pacific regions (13.2%, 95% CI: 10.7%-15.8%). The lowest prevalence of IBS was in the Southeast Asia region (11.0%, 95% CI: 5.8%-17.6%).
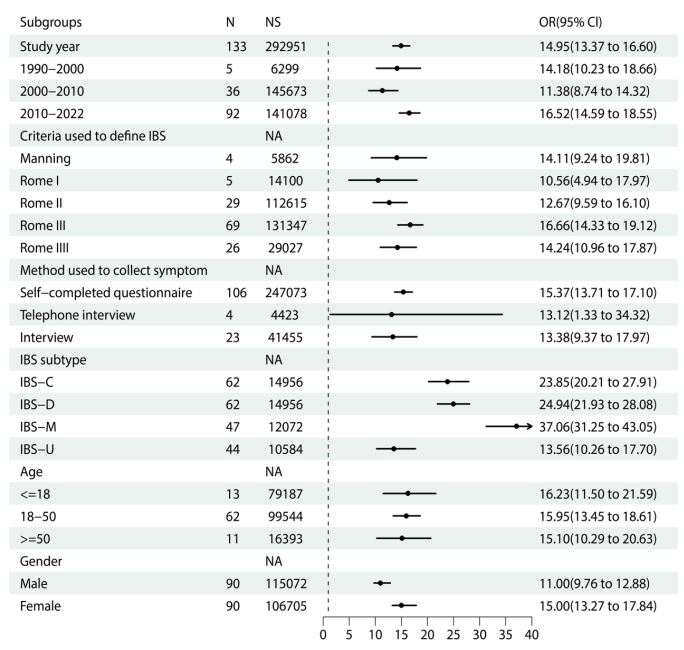
Prevalence of IBS according to study year, criteria used to define its presence and method used to collect symptoms: Of the identified and eligible studies, five studies were conducted between 1990 and 2000, thirty-six studies were conducted between 2000 and 2010, and ninety-two studies were conducted from 2010 onward. The pooled prevalence of IBS was 14.18% (95% CI: 10.23%-18.66%) between 1990 and 2000, 11.38% (95% CI: 8.74%-14.32%) between 2000 and 2010, and 16.52% (95% CI: 14.59%-18.55%) between 2010 and 2022. Meta-regression demonstrated an association between pooled prevalence of IBS and study year (P<0.05), which is shown in Figure 3. In this study, the majority of studies used the Rome III criteria to diagnose the presence of IBS. Only four studies used the Manning criteria, five studies used the Rome I criteria, twenty-nine studies used the Rome II criteria, and twenty-six studies used the Rome IV criteria. The prevalence of IBS was highest when the Rome III criteria were used (16.66%; 95% CI: 14.33%-19.12%) and lowest when the Rome I criteria were used (10.56%; 95% CI: 4.94%-17.97%). The majority of studies used self-completed questionnaires to collect symptoms of IBS. Four studies used the telephone, and twenty-three studies used face-to-face interviews for the survey. Compared with studies that conducted over the telephone or a face-to-face interview, the pooled prevalence of IBS was the highest in studies that used a selfcompleted questionnaire. The pooled prevalence of IBS according to the study year, criteria used to define and method used to collect symptoms is provided in Figure 4.
Prevalence of IBS according to IBS subtype, age and gender: Sixty-two studies reported the predominant stool pattern in the IBS-C and IBS-D subtypes. In these studies, the prevalence of IBS-C and IBS-D was 23.9% (95% CI: 20.2%-27.9%) and 24.9% (95% CI: 21.9%-28.1%), respectively. Forty-seven studies reported that the prevalence of IBS-M was 37.1% (95% CI: 31.3%- 43.1%), and forty-four studies reported that the prevalence of IBS-U was 13.6% (95% CI: 10.3%-17.7%). We also dichotomized the three groups for age according to <=18 years, 18-50 years and >=50 years. Eighty-six studies provided extractable data about age that could be pooled. The pooled prevalence of IBS in those aged 18 years or younger was 16.23% (95% CI: 11.50%-21.59%), in those aged 18-45 years was 15.95% (95% CI: 13.45%-18.61%) and in those aged 50 years or older was 15.10% (95% CI: 10.29%-20.63%). Meta-regression was used to explore the association between the prevalence of IBS and age group. The prevalence of IBS decreased modestly with increasing age in these studies, but none of these differences were statistically significant (P=0.79). According to the gender of the participants, ninety-two studies reported the prevalence of IBS. The pooled prevalence of IBS was higher in women than in men (15.0% [95% CI, 13.3%-17.8%] vs. 11.0% [95% CI: 9.8%-12.9%]), and the OR for IBS in women compared with men was 1.49 (95% CI: 1.35-1.82). The pooled prevalence of IBS according to the IBS subtype, gender and age is provided in Figure 4.
Table 1: Pooled prevalence of IBS by regional and country.
| Region/country | Number of studies | Number of subjects | Pooled prevalence estimate (95% CI) | Heterogeneity I2 |
|---|---|---|---|---|
| African Region | 1 | 2000 | 16.6(15.0,18.3) | - |
| Nigeria | 1 | 2000 | 16.6(15.0,18.3) | - |
| Eastern Mediterranean Region | 28 | 56375 | 17.5(13.8,21.5) | 99.60% |
| Egypt | 1 | 382 | 31.7(27.1,36.4) | - |
| Iran | 7 | 38692 | 10.5(4.4,18.8) | 99.90% |
| Jordan | 1 | 1492 | 13.7(12.0,15.5) | - |
| Lebanon | 2 | 1366 | 20.1(18.0,22.3) | 0.00% |
| Pakistan | 2 | 546 | 31.5(24.8,38.6) | 65.40% |
| Saudi Arabia | 15 | 16156 | 18.5(14.1,23.3) | 97.90% |
| European Region | 29 | 47870 | 15.9(12.7,19.3) | 98.90% |
| Albania | 1 | 502 | 8.6(6.3,11.2) | - |
| Belgium | 1 | 454 | 30.2(26.0,34.5) | - |
| Bulgaria | 1 | 1896 | 20.0(18.2,21.8) | - |
| Croatia | 1 | 703 | 29.2(25.9,32.6) | - |
| Finland | 1 | 3631 | 5.1(4.4,5.8) | - |
| France | 2 | 57813 | 8.5(1.2,21.6) | 99.80% |
| Germany | 2 | 2791 | 16.7(15.3,18.1) | 0.00% |
| Italy | 1 | 653 | 16.9(14.1,19.8) | - |
| Malta | 1 | 192 | 17.7(12.7,23.4) | - |
| Norway | 2 | 4972 | 11.5(5.6,19.2) | 93.60% |
| Palestine | 1 | 1351 | 30.1(27.6,32.5) | - |
| Poland | 1 | 386 | 10.9(8.0,14.2) | - |
| Romania | 1 | 338 | 14.5(11.0,18.4) | - |
| Russia | 1 | 449 | 19.6(16.1,23.4) | - |
| Spain | 2 | 624 | 13.6(9.8,18.0) | - |
| Turkey | 9 | 9543 | 18.2(11.4,26.1) | 97.40% |
| UK | 2 | 3614 | 10.4(0.1,34.9) | 99.70% |
| North America | 15 | 36133 | 14.5(9.0,21.2) | 99.40% |
| Canada | 2 | 6751 | 10.7(0.0,36.5) | 99.80% |
| USA | 13 | 55368 | 15.2(9.1,22.4) | 99.30% |
| South America | 8 | 4834 | 18.9(15.3,22.8) | 87.30% |
| Brazil | 1 | 246 | 19.5(14.8,24.7) | - |
| Chile | 2 | 2275 | 23.2(14.0,33.8) | 95.20% |
| Colombia | 2 | 1603 | 22.1(18.2,26.3) | 72.40% |
| Peru | 3 | 710 | 13.2(10.8,15.8) | 0.00% |
| South-East Asia Region | 9 | 14887 | 11.0(5.8,17.6) | 98.20% |
| Bangladesh | 3 | 6903 | 9.5(5.7,14.1) | 97.10% |
| India | 2 | 4967 | 9.1(0.9,24.7) | 97.20% |
| Indonesia | 3 | 854 | 17.0(2.8,39.7) | 98.10% |
| Sri Lankan | 1 | 2163 | 5.0(4.1,5.9) | - |
| Western Pacific Region | 43 | 130852 | 13.2(10.7,15.8) | 99.00% |
| Australia | 2 | 1158 | 9.7(7.8,11.9) | 27.90% |
| China | 20 | 106341 | 13.0(9.9,16.4) | 99.20% |
| Japan | 7 | 9332 | 16.7(11.5,22.7) | 98.10% |
| Korea | 10 | 12075 | 12.9(6.2,21.7) | 98.10% |
| Malaysia | 4 | 1946 | 10.7(5.5,17.3) | 95.80% |
Discussion
This global large-scale systematic review and meta-analysis provided a comprehensive overview of the prevalence of IBS, which included 131 studies. This meta-analysis extracted data from the available and identified study that reported the prevalence of IBS. The prevalence ranged from 1.1% to 47.5% according to the geographic location, and the pooled global prevalence of IBS was 15.0% (95% CI: 13.4%-16.6%). Most of the studies reported were in the Western Pacific Region, while research in Africa was relatively scarce. Notably, the pooled prevalence of IBS varied strikingly, not only related to the geographic region but also the diagnostic criteria used to define IBS and the investigation method. Besides, the pooled prevalence of IBS was lower with the Rome IV criteria at 14.2%, compared with 16.7% with the Rome III criteria. It has suggested that compared with the Rome III, the stricter criteria of Rome IV could lead to a lower reported prevalence of IBS because fewer individuals would meet the updated criteria. A recent meta-analysis in 2020 reported the similar findings in terms of variability in the prevalence of IBS [21].
In addition, it appeared that the prevalence of IBS might be lower when individuals were interviewed rather than when they were allowed to self-administer the questionnaire. The prevalence of IBS was influenced by the mode of assessment. A recent study directly comparing the prevalence of disorders of gut brain interaction (DGBI) found that DGBI were only half as prevalent when assessed with household vs Internet surveys [25]. While, the finding of this study appears that the effect of the type of assessment is relatively small and the prevalence of IBS might be partly due to underreporting of symptoms when individuals were questioned directly. Moreover, this study showed that the prevalence of IBS has an increasing trend over time from 1990 to 2022, which is consistent with the research results published in 2012 (5). The reasons for the findings are unclear but might be as follows. First, since different criteria have been used over time, it might be accounted for the changes in prevalence of IBS. Second, it has been shown that COVID-19 led to significantly higher prevalence of Functional Gastrointestinal Disorders [22]. The included study in this metaanalysis during the COVID-19 pandemic from 2020 to 2022 are considered to increase the prevalence of IBS. Future epidemiological surveys are needed to provide the prevalence of IBS after the SARS-CoV-2 infection.
Furthermore, our results have showed that the prevalence of IBS in females was slightly higher than that in males worldwide [26-28]. The pooled prevalence of IBS was 15.0% in females and 11.0% in males. In contrast to the previous clinical research, this study seems to reveal only a small difference between males and females. Because the population-based studies included in this review may include subjects with ‘organic’ causes of the IBS-type symptoms. Besides, the pooled prevalence of IBS in those aged 18 years or younger was 16.23%, that in those aged 18-50 years was 15.95% and that in those aged 50 years or older was 15.10%. Thus, the prevalence of IBS appeared to decline modestly with increasing age. Similar to our findings, these studies showed that the prevalence of IBS varied according to age group [29-31]. When the predominant stool pattern with IBS was examined, IBS-M was the most prevalent, which was consistent with this study reported [32]. Meanwhile, IBS-U was the least prevalent and the difference was not statistically significant.
There are several limitations of this study. First, with limited generalizability considering the lack of data in some regions and countries, this study only reported 39 countries globally. Second, although the sources of heterogeneity were explored, there was significant heterogeneity between studies when data were pooled in almost all instances. It was still limited by the lack of information in the original studies, including age, sex, and subtype of IBS. This heterogeneity was not explained by any of the subgroup analyses we conducted. Finally, it should be noted that the diagnostic criteria for IBS in this study do not necessarily equate to a definitive diagnosis of IBS. There might be other organic conditions, including celiac disease, small intestinal bacterial overgrowth, or inflammatory bowel disease, that mimic IBS or lead to similar symptoms. In conclusion, more future epidemiological research reporting the prevalence of IBS in the general population is needed, in particular the SARSCoV-2 infection.
Declarations
Author contributions: LL contributed to the conception and design of the study. LCP and YYY participated in literature searching, literature screening, data extraction and writing work. ZY and LX participated in quality assessment, data analysis and writing work. LL approval of final manuscript. All the authors read and approved the final version of the manuscript.
Funding: No Funding.
Data availability: The datasets and materials analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for publication: Not applicable.
Ethics approval and consent to participate: Not applicable.
Competing interests: No potential conflict of interest relevant to this article was reported.
References
- Sebastián Domingo JJ: Irritable bowel syndrome. Med Clin (Barc). 2022; 158(2): 76-81.
- Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016; 1(2): 133-146.
- Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. 2015; 313(9): 949-958.
- Sultan S, Malhotra A. Irritable Bowel Syndrome. Ann Intern Med. 2017; 166(11): 81-96.
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012; 10(7): 712-721. 714.
- Camilleri M: Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. 2021; 325(9): 865-877.
- Takeoka A, Kimura T, Hara S, Hamaguchi T, Fukudo S, Tayama J. Prevalence of Irritable Bowel Syndrome in Japan, China, and South Korea: An International Cross-sectional Study. Journal of neurogastroenterology and motility. 2023; 29(2): 229-237.
- Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016; 2: 16014.
- Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J Gastroenterol. 2014; 20(22): 6725-6743.
- Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012; 107(7): 991-1000.
- Payne S. Sex, gender, and irritable bowel syndrome: making the connections. Gend Med. 2004; 1(1): 18-28.
- Qin HY, Cheng CW, Tang XD, Bian ZX. Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol. 2014; 20(39): 14126-14131.
- Ford AC, Lacy BE, Talley NJ: Irritable Bowel Syndrome. N Engl J Med. 2017; 376(26): 2566-2578.
- Adriani A, Ribaldone DG, Astegiano M, Durazzo M, Saracco GM, Pellicano R. Irritable bowel syndrome: the clinical approach. Panminerva Med. 2018; 60(4): 213-222.
- Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020; 17(8): 473-486.
- Liu YL, Liu JS. Irritable bowel syndrome in China: a review on the epidemiology, diagnosis, and management. Chin Med J (Engl). 2021; 134(12): 1396-1401.
- Saha L: Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014; 20(22): 6759-6773.
- Grayson M: Irritable bowel syndrome. Nature. 2016; 533(7603): 101.
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021; 160(1): 99-114.e113.
- Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. 2017; 66(6): 1075-1082.
- Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020; 5(10): 908-917.
- Zhang D, Chen C, Xie Y, Zeng F, Chen S, Chen R, Zhang X, Huang S, Li D, Bai F. Post-infection functional gastrointestinal disorders following coronavirus disease-19: a prospective follow-up cohort study. BMC Infect Dis. 2023; 23(1): 422.
- Golla R, Vuyyuru S, Kante B, Kumar P, Thomas DM, Makharia G, Kedia S, Ahuja V. Long-term Gastrointestinal Sequelae Following COVID-19: A Prospective Follow-up Cohort Study. Clin Gastroenterol Hepatol. 2023; 21(3): 789-796.e781.
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012; 65(9): 934-939.
- Sperber AD, Bor S, Fang X, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL et al. Faceto-face interviews versus Internet surveys: Comparison of two data collection methods in the Rome foundation global epidemiology study: Implications for population-based research. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2023; 35(6): 14583.
- Kim YS, Kim N. Sex-Gender Differences in Irritable Bowel Syndrome. Journal of neurogastroenterology and motility. 2018; 24(4): 544-558.
- AlButaysh OF, AlQuraini AA, Almukhaitah AA, Alahmdi YM, Alharbi FS. Epidemiology of irritable bowel syndrome and its associated factors in Saudi undergraduate students. Saudi journal of gastroenterology: official journal of the Saudi Gastroenterology Association. 2020; 26(2): 89-93.
- Keshteli AH, Dehestani B, Daghaghzadeh H, Adibi P. Epidemiological features of irritable bowel syndrome and its subtypes among Iranian adults. Annals of gastroenterology. 2015; 28(2): 253-258.
- Endo Y, Shoji T, Fukudo S. Epidemiology of irritable bowel syndrome. Annals of gastroenterology. 2015; 28(2): 158-159.
- Lai YT, Chen CY, Bair MJ. Epidemiology, Clinical Features, and Prescribing Patterns of Irritable Bowel Syndrome in Taiwan. Front Pharmacol. 2021; 12: 788795.
- Trindade IA, Melchior C, Törnblom H, Simrén M: Quality of life in irritable bowel syndrome: Exploring mediating factors through structural equation modelling. Journal of psychosomatic research. 2022; 159: 110809.
- Su AM, Shih W, Presson AP, Chang L. Characterization of symptoms in irritable bowel syndrome with mixed bowel habit pattern. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2014; 26(1): 36-45.