Research ArticleOpen Access, Volume 2 Issue 1
Human Immunodeficiency Virus Treatment Roll-Up: Challenges for Military Health Facilities in Cameroon in Sample Collection for Viral Load Testing and Early Infant Diagnosis
Tatiana Noumi1,2*; Marie P Yede1,3; O’Neal Youte1 ; Nadège Maru1 ; Ethel Shang1,4; Clinton Fatcheu3 ; Melissa Ngouega3 ; Ubald Tamoufe2 ; Julius Nwobegahay1
11Military Health Research Center (CRESAR), Cameroon.
2Health and Development in Action (HEADA), Cameroon.
3University of Yaoundé I, Cameroon.
4University of Buea, Cameroon.
*Corresponding author: Tatiana Noumi
Military Health Research Center (CRESAR), Cameroon.
Received : Jan 27, 2024 Accepted : Feb 13, 2024 Published : Feb 20, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Noumin T © All rights are reserved
Citation: Noumi T, Yede MP, Youte O, Maru N, Shang E, et al. Human Immunodeficiency Virus Treatment Roll-Up: Challenges for Military Health Facilities in Cameroon in Sample Collection for Viral Load Testing and Early Infant Diagnosis. Epidemiol Public Health. 2024; 2(1): 1032.
Abstract
HIV viral load testing is an important element in the HIV elimination process, as it enables the efficacy of treatment to be assessed and, consequently, the patient’s viral suppression process to be monitored. A number of factors can affect the results of viral load tests, distorting patient outcomes and affecting follow-up. In developing countries, these challenges, linked to the technical platform, are much more likely to be encountered in the health facilities responsible for the pre-analytical phase, and can therefore compromise sample integrity. The aim of the study was to assess the challenges faced by Cameroon’s military health facilities in collecting samples for viral load and early infant diagnosis. A survey was carried out to 22 military health facilities to supervise sample collection activities using a specific grid. Data were recorded into the Google forms interface and followed by analysis using Microsoft office Excel 2013. 95% of facilities had a long working experience of over two (02) years for each type of handling. Only 27% of facilities used blood sampling protocols to the vacuum system in the HIV-VL test. During the survey, 18% of sites did not have a centrifuge and used decantation at room temperature. Additionally, 64% of health facilities did not fill in temperature control sheets properly, 23% jeopardize the cold chain due to insufficient cold accumulators or poorly maintained coolers. Only eight (08) health facilities did a HIV early infant diagnosis testing. The required technical platform was not present in all health facilities, and staff technical skills in charge of the pre-analytical stages were still not as high as expected. This is a call to strengthen the capacity of sample collection sites to achieve the WHO’s goal of eliminating HIV transmission by 2030.
Keywords: Challenges; HIV viral load testing; HIV early infant diagnosis; Specimen integrity; Military health facilities.
Introduction
The vision of UNAIDS is to achieve, by 2030, viral suppression in 95% of People Living with HIV (PLHIV) under antiretroviral therapy [1]. To this end, viral load testing is the main tool for managing patients on ART, optimizing its efficacy and preventing the build-up of viral resistance that can compromise future treatment lines. Consequently, obtaining the correct values depends on the quality of the entire testing process, including the three phases of biological analysis: pre-analytical, analytical and post-analytical.
In developing countries where the burden of HIV is high, achieving this “good quality” is affected by various challenges centered on the resources put in place to meet the growing rates of people being monitored and to accommodate the remote residence of some (Global AIDS Strategy 2021-2026, Ending Inequalities, Ending AIDS, n.d.). Improvements are constantly being made, and there are already several reference laboratories in these countries, equipped and supplied to carry out HIV tests. However, the pre-analytical phase still faces challenges affecting and reducing test quality [2,1] (Newman H, 2020; UNAIDS, 2023; Key benchmarks on HIV/AIDS, n.d.). The health facilities involved in this pre-analytical phase must therefore respond to the challenges of sample collection, pre-treatment, storage, transport and documentation [2], which must meet the requirements of good laboratory practice while adapting to the work context.
In Cameroon, the Military Health Research Center (CRESAR) is one of a number of facilities authorized, equipped and supplied to HIV Viral Load (HIV-VL) and Early Infant Diagnosis of HIV (EIDHIV) tests. The handled samples (plasma-ETDA and dried blood drops) come from a range of collection sites in military and civilian health facilities. Therefore, to ensure that collected specimens have preserved all their integrity, CRESAR made a supervision visit to some of military health facilities in Cameroon. During this activity, a survey was carried out on the current state of technical facilities and staff practical skills involved in this preanalytical phase. The survey results are presented below, and revealed many of the health facilities’ strengths, as well as areas for improvement and challenges they face.
Materials and methods
From 18 to 29 April 2023, a visit was carried out to 22 Military Health Facilities (MHF) in nine (09) regions of Cameroon to supervise sample collection activities. These facilities (Table I) were located in 04 joint military regions and 08 military health sectors. They are responsible for collecting, preparing, storing and shipping samples to the Military Health Research Center (CRESAR) for viral load testing and early infant diagnosis of HIV. Authorization N°23000265/MRP/MINDEF/026 dated March 30th, 2023 from the Ministry of Defense via the Military Health Division of Cameroon was obtained prior to the field visit. The purpose of the visit was twofold: (1) to assess the technical facilities and practical capabilities of the staff, and (2) to provide initial support in building the capacity of these training facilities.
CRESAR scientists carried out a cross-sectional descriptive survey. The survey was done using a specially designed supervision grid. Supervisors based on observations, information received and documents supplied completed this grid. Data collected in all the supervised health facilities was entered into the Google forms interface and analyzed using Microsoft office Excel 2013.
We then provided documentation that was constantly useful for the site, as well as mini-training designed to correct any shortcomings observed on site. This documentation included standard operating procedures for on-site sample collection and processing, practical guides for sample collection and transport, temperature monitoring sheets for refrigerators and freezers, patient information sheets and packing slips. A mini-training session consisted of a reminder of good working practices, combined with negative answers to the information collection questions.
Table 1: Military health facilities visited.
| Regions | Adamawa (01) |
Center (05) | East (02) | Far North (01) |
Littoral (05) | North (01) | South (03) | South-West (02) |
|---|---|---|---|---|---|---|---|---|
| Health facilities | Military health sector 5 |
1st Region Military Hospital |
Bertoua Military Hospital |
4th Region Military Hospital |
2nd Region Military Hospital |
3rd Region Military Hospital |
Ebolowa Military Hospital |
Military Medical Center of the Special Amphibious Battalion |
|
Military Medi- cal Center of the Presidential Guard |
Military Medical Center of Abong- Mbang |
Military Medi- cal Center of the Surface-Surface Artillery Regiment |
Military Medi- cal Center of Kribi |
Military Medi- cal Center of Buea |
||||
|
Military Medi- cal Center of the Headquarters Brigade |
Military Medi- cal Center of the Surface-Air Artillery Regiment |
Military Medi- cal Center of Djoum train- ing center |
||||||
|
Military Medical Center of the Air Force Base |
Military Medical Center of the Ar- mored Reconnais- sance Battalion |
|||||||
|
Military Medi- cal Center of the State Secretary for Defense |
Military Medi- cal Center of the Military Engineering Regiment |
|||||||
|
Military Medi- cal Center of the Nkoteng Squadron Group of Gendar- merie |
Firefighters' Medi- cal Center |
Table 2: Evaluation of criteria associated with the storage and dispatch of samples by MHF.
| Elements of assessment | Observations | |||||
|---|---|---|---|---|---|---|
| Yes | Partially | No | ||||
| n | % | n | % | n | % | |
| A freezer (-20°C) is used to store samples. | 22 | 100 | 0 | 0 | 0 | 0 |
| Freezer temperature is continuously monitored and recorded. | 7 | 32 | 1 | 4 | 14 | 64 |
|
A sufficient number of cold accumulators
(>10) are stored (-20°C) in case of load shedding. |
15 | 68 | 5 | 23 | 2 | 9 |
| The unit has coolers in good condition for transporting samples. | 17 | 77 | 5 | 23 | 0 | 0 |
| A sufficient number of cold accumulators/ice pack are used for transport. | 20 | 91 | 2 | 9 | 0 | 0 |
| Samples are transported in a cooler in good condition. | 21 | 95 | 1 | 5 | 0 | 0 |
| The triple packaging system is respected. | 22 | 100 | 0 | 0 | 0 | 0 |
| Sample volumes are always respected. | 20 | 91 | 2 | 9 | 0 | 0 |
| Samples arrive chilled or frozen at CRESAR. | 22 | 100 | 0 | 0 | 0 | 0 |
Results
Observation of pre-analytical processes for HIV viral load
Of the sites supervised, one (01) had more than six (06) months’ experience (but less than 1 year) in handling specimens for HIV viral load testing, and the others had more than two (02) years’ experience. It was found that staff involved in handling are constantly updated on sample collection and processing procedures.
Obtaining samples
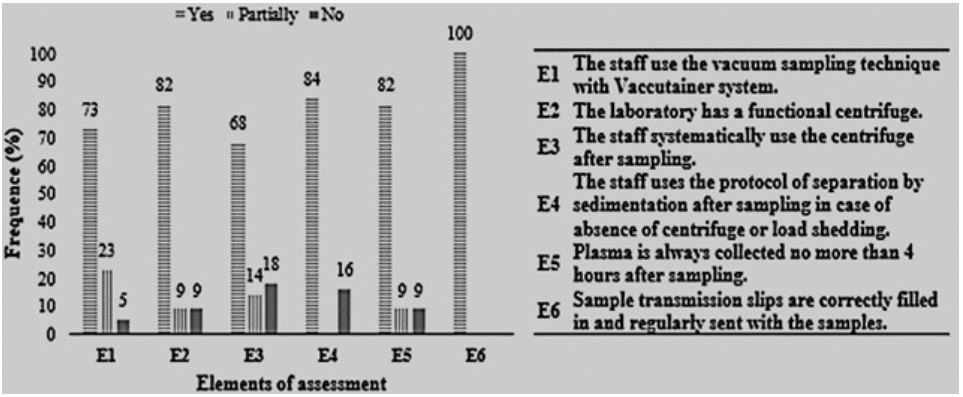
Most MHF work in such a way as to minimize poor-quality samples. Observations concerning sample procurement are summarized in Figure 1.
From analysis of Figure 1, blood collection techniques and plasma collection times were found to be different at some sites compared with the majority of collection sites. It was observed that some sites were faced with a material limitation (availability of a functional centrifuge) to obtain optimal specimen, blood plasma. However, it was noted that for some sites, this limitation or load shedding did not prevent staff from working, thanks to the use of the blood decanting technique.
Sample storage and transport
During the survey, it was found that the equipment and electrical circuits were supplied with electric power (95%) and solar power. Energy supplies were continuous for 45% of health facilities, interrupted but stable for 36% and interrupted but unstable for 13%. In addition, 45% of health facilities had a functional secondary generator. For facilities with interrupted supplies, supply frequencies ranged from two (02) times a day to four (04) times a week.
Samples stored in Cryo-tubes were catalogued and sent to CRESAR twice a week. Identified transport times from collection sites to CRESAR were 03 days (Adamwa, North, Far North), 01 day (East, Littoral, South, South-West, Center/Nkoteng). Specimens from other sites (Center) are collected in one day by CRESAR. Other observations concerning sample conservation are summarized in Table 2. It was noted that some sites had material limitations during the survey (insufficient number of cold accumulators and coolers in good condition). It can be seen from this table that the sites have a system in place to ensure the proper preservation and integrity of samples on site and during transport, so that the transport times, load shedding and material limitations observed do not affect the samples received by CRESAR (samples arrive refrigerated or frozen at CRESAR).
Observation of pre-analytical processes for HIV early detection
Only eight (08) sites were recorded as handling specimens for early infant detection of HIV testing. They all had more than two (02) years’ experience in such handling. Of these sites, 75% had mastered the art of collecting blood on absorbent paper during the survey. Also, after sampling, the drying of the blood on the absorbent paper was carried out as planned in 87% of the facilities, i.e. the blood deposited on the blotting paper was left to dry in the correct environment (clean, dust-free, in the shade) for 24 hours. As with the HIV-VL samples, each site avoided the risk of confusing the samples it sent for analysis. Dried blood drop specimens were forwarded to CRESAR using a triple-pack system at the same time as HIV plasma samples, and did not present any sample rejection criteria on arrival.
Discussion
The aim of this survey was to evaluate the technical platform and practical skills of staff involved in the pre-analytical stages of viral load testing and HIV early infant diagnosis in in military health facilities in Cameroon. To this end, a survey was carried out in twenty-two (22) military health facilities, which revealed a number of strengths and areas for improvement.
First and foremost, it was found that all the facilities were involved in handling specimens for HIV testing, and only eight (08) in those for HIV early infant diagnosis testing. With the exception of one (01) site with six (06) months’ experience in handling specimens for HIV testing, all the facilities had a long working experience of over two (02) years for each type of handling. In this experience, we can identify adaptation to the field through the time already spent working, as well as the training these facilities receive as part of the HIV programs set up in Cameroon. This adaptation and training would justify the strengths and flexibility of the work observed during the survey, despite the challenges of certain sites.
Among the areas for improvement identified by 27% of facilities was the use of alternative blood sampling protocols to the vacuum system in the HIV-VL test. This may include syringeonly sampling, or a combination of winged needle and syringe. This adds a blood transfer step into EDTA tubes, which not only increases the risk of infection for personnel, but also increases the risk of reduced sample integrity due to potential blood hemolysis. However, blood hemolysis is a cause of false low viral load values, as it affects nucleic acid extraction and PCR [2].
After collection, the plasma is obtained. The maximum time between collection of the whole blood sample and separation of the plasma is 6 to 24 hours at room temperature and 48 hours at 2 to 8°C (Newman, World Health Organization, 2019). Also, when centrifugation is not possible, this time should not exceed 6 hours [2]. However, a maximum of 4 hours in the absence or presence of a centrifuge has been recommended by CRESAR to avoid forgetting and ensure better specimen integrity, although 18% do not follow this recommendation. What’s more, in the event of a power cut, others (16%) with a working centrifuge preferred to wait for power to return before centrifuging blood samples. This presents a risk of exceeding plasma separation times. Prolonging the storage time of a sample before plasma separation could have adverse consequences on the performance of the test and exposes clinicians and patients to the risk of incorrect results [2]. Nevertheless, facilities are constantly reminded to respect this time and not to exceed 24 hours if a case of forgetfulness arises, in order to avoid any erroneous results, since any interaction between the RNA’s released by the cells and the viral RNA must be avoided [2].
In the field, plasma is obtained by centrifugation, ideally, and by decantation during periods of peak demand. However, it was found that 18% of sites did not have a centrifuge during the survey, forcing sites to use decantation at room temperature. Some studies have shown that the viral load was more accurate when centrifuges were used [3,4]. According to these authors, this centrifugation-dependent increase in viral load results from a “leakage” of HIV nucleic acids. The proviral DNA and intracellular HIV RNA present in the cellular component of whole blood arrived in the plasma during centrifugation, making the viral load more accurate than during separation by decantation. These observations only prove the importance for all health facilities to use a centrifuge during the pre-analytical process of samples intended for HIV viral load measurement this for more reliable results.
Most facilities are far from CRESAR. This imposes a continuous energy supply for good preservation to avoid RNA degradation and falsely low HIV-VL values [2]. However, it has been found that 55% of supervised facilities do not have a continuous energy supply, and 55% do not have a functional secondary energy supply. Such situations are risk factors for poor preservation and lack of sample centrifugation, and have already been reported by various authors for developing countries [2].
Although the presence of refrigerated containers in health facilities is important, they can be useless if they are poorly used and/or maintained. Temperature monitoring is the key to logistics that respect the cold chain. It enables temperature curves to be monitored in real time so that action can be taken to avoid risks that could lead to a break in the cold chain this thanks to the completion of temperature sheets [5]. In this survey, it was found that 64% of health facilities did not fill in temperature control sheets properly. This finding raises questions about the reasons for this failure, which may be answered by a lack of training on how to fill in the said sheets, or by laxity on the part of staff.
Cold protects nucleic acids from the enzymatic degradation and microbial proliferation that can occur at room temperature [6]. A good cold chain system enables these temperatures to be respected, as a well-maintained cold chain implies very precise adherence to sample preservation temperatures during storage and transport. However, it has been found that 23% of health facilities jeopardize the cold chain due to insufficient cold accumulators or poorly maintained coolers. As many studies have shown, respect for the cold chain protects samples against thermal lysis and reduces biological and chemical reactions in the sample (Cryopreservation and its clinical applications - PMC, n.d.) [7]. It could therefore be argued that the cold chain in some health facilities presents a high risk of negatively influencing sample quality and, consequently, the viral load result obtained, and this suggests that the material resources of health facilities should be strengthened.
It was found that health facilities mainly (95%) use their coolers in good condition, and that samples arrive at CRESAR refrigerated or frozen. This suggests that, despite the limitations, health facilities are concerned to guarantee the integrity of the samples collected and transmitted. In addition, the “freezer - cold accumulator” combination offers these sites an alternative solution for ensuring the integrity of samples during on-site storage in the event of load shedding for a period which, in the field, proved to be relatively short (maximum 03 days after sampling). However, this should not be a definitive solution, since the primary involvement of cold accumulators is in transport, and such a solution would make them insufficient for this role.
While the storage of plasma samples can sometimes prove difficult, due to the often poor maintenance of the cold chain between healthcare facilities and the structures where the tests are carried out, the use of dried blood drop samples on blotting paper for HIV testing presents a different picture. This is due in particular to the absence of a requirement for the use of centrifuges and a cold chain, since storage and transport can be carried out at room temperature [2]. Of the eight (08) health facilities that took blood samples on blotting paper for HIV testing of newborns, 75% had a good command of blood sampling on absorbent paper, and 87% dried the blood properly after sampling. In view of all these advantages and the urgent need to diagnose children exposed to HIV at birth, it is urgent to implement blotting paper sampling in all health facilities and to train as many staff as possible. In view of these advantages, HIV testing protocols using dried blood would be a great asset in the current context. In addition, there is a risk of infection when transporting this type of sample.
These points for improvement are noted as being the challenges constantly encountered in resource-limited areas [2]. But in view of the current results in Cameroon, this survey shows that a great deal of work has already been done at the sample collection sites, despite these barriers. As a result, strengthening these sites and providing solutions to the limitations encountered would be a booster for improving the work and another step towards achieving the goal of HIV eradication.
Conclusion
With the expansion of treatment monitoring approaches involving viral load and infant diagnostic tests, it is essential to carry out regular supervisions in the various care centers responsible for the pre-analytical stages, to guarantee the reliability of results and the success of the programs. The aim of this survey was to take stock of the technical platforms and practical capabilities of military health facilities involved in the pre-analytical stages of viral load and early HIV diagnosis in newborns in Cameroon. At the end of this survey, which was carried out in 22 military health facilities, it was observed that the required technical platform was not present in all health facilities, and that the technical capabilities of the personnel in charge of the preanalytical stages were still not as high as expected. This is a call to strengthen the capacity of sample collection sites to achieve the WHO’s goal of eliminating HIV transmission by 2030.
Declarations
Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding source: The survey was financially supported by DHAPP/PEPFAR Cameroon.
Data availability statement: Data associated with this work were not deposited into a publicly available repository. Data included in article/supp. material/referenced in article.
Acknowledgements: The authors would like to thank DHAPP/PEPFAR Cameroon, the Military Health Research Center (CRESAR) and the HEADA association for their support throughout this survey. The authors also thank the military health units for their participation.
References
- ONUSIDA. Fiche d’information-Dernières statistiques sur l’état de l’épidémie de sida | ONUSIDA. 2023. https://www.unaids.org/fr/resources/fact-sheet
- Newman HHD. HIV-1 viral load testing in resource-limited settings: Challenges and solutions for specimen integrity. Rev Med Virol. 2020 ;https://doi.org/10.1002/rmv.2165
- Giordano MKT. The effects of the Roche AMPLICOR HIV- 1 MONITOR UltraSensitive Test versions 1.0 and 1.5 viral load assays and plasma collection tube type on determination of response to antiretroviral therapy. 2006.
- Griffith BP. Increased levels of HIV RNA detected in samples with viral loads close to the detection limit collected in plasma preparation tubes (PPT). J Clin Virol. 2006; 35:197-200.
- koovea. Surveillance de température et tracabilité des tempèratures des refrigérateurs:3 inormations à retenir. Récupéré sur. 2019. https://www.koovea.com/surveillance-chaine-du-froidtempérature-refrigerateurs
- Tae Hoon Jang aS, Tae H. Cryopreservation and its clinical applications. National Librery of Medecine. 2017.
- Cryopreservation and its clinical applications-PMC. (s. d.). Consulté 1 janvier 2023, à l’adresse https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5395684/.