Research ArticleOpen Access, Volume 2 Issue 1
Is There a Window of Opportunity to Treat Early Onset Obesity with Omega-3-Fatty Acids?
Reiner Buchhorn*
Praxis for Pediatrics, Pediatric Cardiology and Adults with Congenital Heart Disease, Am Bahnhof 1, 74670 Forchtenberg, Germany.
*Corresponding author: Reiner Buchhorn
Praxis for Pediatrics, Pediatric Cardiology and Adults with Congenital Heart Disease, Am Bahnhof 1, 74670 Forchtenberg, Germany.
Received : Nov 23, 2023 Accepted : Jan 18, 2024 Published : Jan 25, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Buchhorn R © All rights are reserved
Citation: Buchhorn R. Is There a Window of Opportunity to Treat Early Onset Obesity with Omega-3-Fatty Acids?. Epidemiol Public Health. 2024; 2(1): 1028.
Abstract
Early onset obesity has a negative impact on later life obesity and an enhanced cardiovascular risk. After showing a significant beneficial effect of omega 3-fatty supplementation in 38 children with an age >6 years on elevated heart rate in obese children without an improvement of the body mass index in later childhood in a recent publication, we now include the data of 6 children with an age <6 years who received 50 mg/kg Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) as a suspension per day.
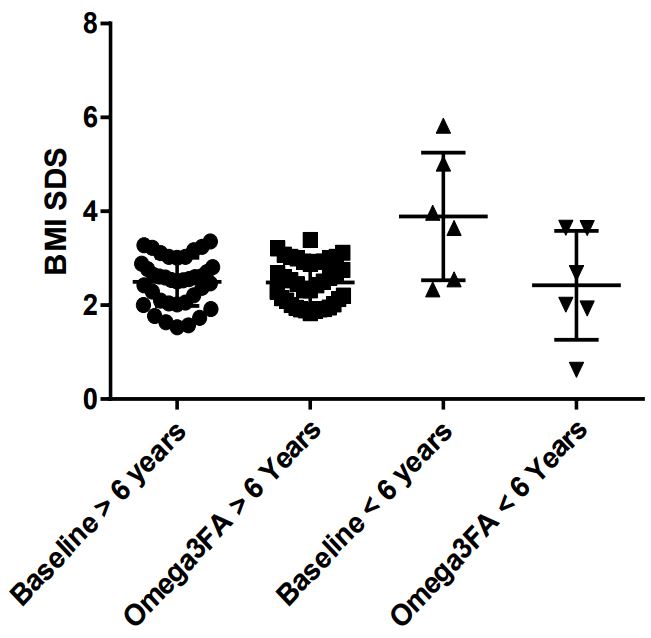
Results: The data confirm the effect of omega-3-fatty acid supplementation on 24-hours heart rate (97.0±14.9 to 91.3±13.5 bpm; p=0.0082) with an additional beneficial effect on the Body mass index SDS (from 2.7±0.8 to 2.5±0.6; p=0.0354) that depends on the 6 children with an age <6 years, as illustrated in a two case reports, including one boy with biallelic leptin receptor deficiency.
Conclusion: With respect to the growing number of obese children and the impact of obesity on mortality in the COVID-19 pandemic, the possibility to improve this urgent problem with a low cost and low risk intervention with omega-3-fatty acid supplementation in children with early onset obesity should be proofed in a prospective randomized trial.
Introduction
The prevalence of childhood obesity remains very high, and despite intensive efforts to stop this pandemic, the therapy results are disappointing. Recent observational studies indicate that the most rapid weight gain occurred between 2 and 6 years of age and persist or often increase until adolescence [1] and adulthood. We further have to realize that mortality in the COVID-19 pandemic was related to obesity [2] and the pandemic have a further negative impact on children’s weight gain [3].
Mortality in obese children is related to the metabolic syndrome with cardiovascular endpoints starting in the late twenties in life [4]. Primary prophylaxis in early childhood would be a successful strategy to prevent obesity and its detrimental consequences in later life.
The increase in resting heart rate and mean 24-hours mean heart rate is one of the first indicators of the ongoing cardiovascular risks in adolescents as shown in an observational study from Sweden [5]. We proof that increasing mean heart rates and the so-called inappropriate sinus tachycardia often occur in obese children and can be improved with omega-3-fatty supplementation [6]. This significant therapeutic effect of omega-3-fatty supplementation is more pronounced in children compared to a metanalysis in adults [7] and depends on the baseline heart rate: The higher the baseline heart rate prior to supplementation the higher the heart rate lowering effect of omega-3-fatty acid supplementation [8]. This effect on an important cardiovascular risk factor is highly significant but we found no beneficial effect on the elevated body mass index on average in our first cohort [9].
We recently start omega-3-fatty acid supplementation in early onset obesity, if the infants or toddlers have elevated heart rate and/or blood pressure. Our current analysis in 44 children with an age between 9 month and 18 years indicate an impressive beneficial effect of omega-3-fatty acid supplementation on the body mass index in early childhood.
Patients: The current study included 44 European children and adolescents with a BMI above the 95th percentile and an average age of 11.0 years (patient characteristics are summarized in Table 1). All patients were referred to the pediatric outpatient clinic of the Caritas Hospital Bad Mergentheim or my private praxis in Forchtenberg for further diagnostics and therapy for obesity. All study participants and their parents or guardians provided informed consent.
Design: Obese children and adolescents were screened for autonomic dysfunction by 24-h Holter ECG analysis. In addition to implementing diet and lifestyle changes, we recommended that our patients should use omega-3 fatty acid supplements. Patients usually purchased different products based upon 1-2 g fish oil per day from a retail store. The following dose recommendations were given: Children up to an age of 8 years should receive at least 50 mg/kg Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) as a suspension per day. Children who were able to swallow capsules should receive at least 800 mg EPA and DHA per day. We performed a second analysis after 145±113 days omega-3-fatty acid supplementation.
24-hour ECG: To record ECG results, we used a two-channel Holter monitor (Pathfinder; Spacelabs Healthcare GmbH, Nürnberg, Germany). Children and adolescents were not placed on bed rest; instead, they followed their usual daily routines. The Holter ECG recordings were analysed as average values of all data collected during 24 h. For the current analysis we only use the 24-hours mean heart rates, if analysis of heart rate variability is separately published four years ago [9].
Statistical analysis: We used SPSS version 23.0 for all statistical analyses. All results were reported as mean ± standard deviation (SD) and standard error of mean (SEM). Parametric statistics were used for all comparisons because most variables were normally distributed. Patients were compared before and after omega-3 fatty acid supplementation using a paired samples t-test for equality of means. A p-value<0.05 was considered statistically significant.
Results
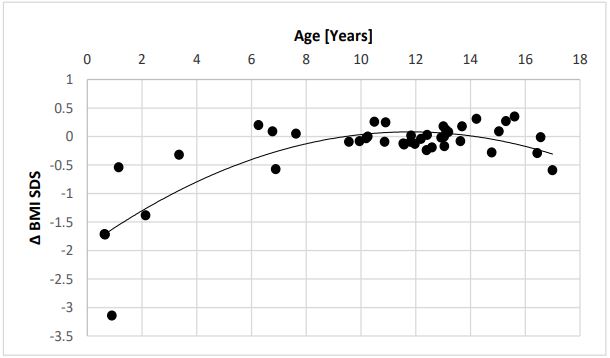
Our data, displayed in Table 1, confirm the highly significant effect of omega-3-fatty supplementation on elevated heart rates in obese children from 97.0±14.9 to 91.3±13.5 bpm (p=0.0082). By including young children with early obesity, the body mass index slightly decreases from 29.4±5.0 to 28.9±5.4 kg/m2 and the BMI-SDS significantly decrease from 2.7±0.8 to 2.5±0.6 (p=0.0354) (Figure 1). This effect most of all depends on the young children with an age <6 years (Figure 2).
Table 1: Patient data at Baseline and after 145 days on average Omega-3-Fatty Acid Supplementation.
| Baseline | Omega-3-Fatty Acid Supplementation | P-Value | |||
|---|---|---|---|---|---|
| N=44 | Mean | SD | Mean | SD | |
| Age [Years] | 11.0 | 4.7 | 11.3 | 4.6 | ns |
| Duration [days] | 145.3 | 112,6 | |||
| Height [cm] | 148 | 30 | 150 | 28 | ns |
| Height Percentile [%] | 64.7 | 30.0 | 66.7 | 30.6 | ns |
| Height SDS | 0.83 | 2.12 | 0.66 | 1.27 | ns |
| Weight [kg] | 69.2 | 30.2 | 70.1 | 29.4 | ns |
| Weight Percentile [%] | 97.2 | 5.0 | 96.7 | 6.0 | ns |
| Body Mass Index [kg/m2] | 29.4 | 5.0 | 28.9 | 5.4 | ns |
| Body Mass Index Percentile [%] | 98.7 | 1.5 | 98.2 | 4.0 | ns |
| Body Mass Index SDS | 2.7 | 0.8 | 2.5 | 0.6 | 0.0354 |
| Sys. BP [mmHg] | 126 | 15 | 126 | 10 | ns |
| Diast. BP [mmHg] | 69 | 11 | 67 | 11 | ns |
| Heart Rate [bpm] | 97 | 15 | 91 | 13 | 0.0082 |
Sys./Diast. BP: Systolic and Diastolic Blood Pressure.
Case reports
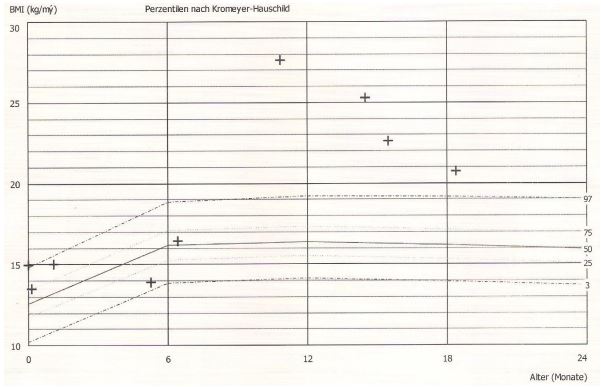
Case 1: The 10-month-old girl was presented from her parents after she had gained weight up to 17 kg after starting to feed complementary foods (Figure 3). Laboratory shows elevated IGF-1 (156 μg/l) and IGF-BP-3 (5.14 mg/l) values. The leptin value is in the normal range (21 μg/l). A hypophysis tumour was excluded by cranial MRI. The blood pressure was elevated and we start 50 mg/kg omega-3-fatty acid supplementation (MensSana Omega-3 liquid™, Forchtenberg, Germany). Within the following 12 month, the girl had no further weight gain and while growing on the 97% percentile the body mass index decreases from 27.6 to 20.7 kg/m2 . The IGF-1(83 μg/l) and IGF BP3 (2.2 mg/l) values decrease in the normal range.
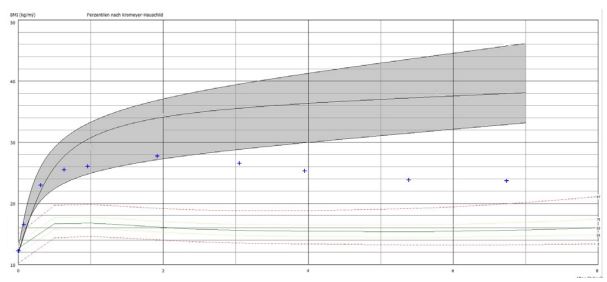
Case 2: The 12-month-old boy was presented from his parents after he had gained weight up to 19 kg (Figure 4). A hypophysis tumour was excluded by cranial MRI. Genetic diagnostics at the university hospital Ulm shows biallelic leptin receptor deficiency (E66.85, OMIM 601007). The blood pressure was elevated and we start 50 mg/kg omega-3-fatty acid supplementation (MensSana Omega-3 liquid™, Forchtenberg, Germany). Within the following 4 years the body mass index decreases from 27.7 to 23.7 kg/m2 and the heart rate and blood pressure normalize.
Discussion
Our data clearly demonstrate again a significant effect of omega-3-fatty acid supplementation on the elevated heart rates (97,0±15.0 to 91,3±13,5bpm, p=0.0082) in obese children as recently published [9]. By including children with early onset obesity, we further demonstrate a significant effect on the SDS Score of the body mass index (2,69±0,82 to 2,48±0,58, p=0.0354) in contrast to our first cohort of older children. This effect most of all depends on the 6 youngest children between 9 month and 5 years (Figure 1+2). We further demonstrate this effect in a 10-month-old girl with a bodyweight of 17 kg and a body mass index of 27.6 kg/m2 , 5.82 SDS over the 50% percentile (Case 1). After omega-3-fatty acid supplementation the highly elevated bodyweight show a weight arrest for one year and the body mass index decrease from 27.6 to 20.7 kg/ m2 without no further therapeutic intervention. During omega3-fatty supplementation the elevated IGF-1 level decrease from 156 μg/l to 83 μg/l within 3 months.
Case 2, as illustrated in Figure 4, shows the body mass indices up to 7 years of age of a boy with severe early obesity due to monogenetic obesity of cause a leptin receptor deficiency treated with Omega-3-Fatty acids since the age of 3 years. The body mass index decreases from 27.7 to 23.7 kg/m2 clearly below the published early childhood body mass index trajectories for this monogenetic obesity [10].
Despite many publications, omega-3-fatty acid supplementation for cardiovascular risk prophylaxis is still under discussion. Current research focussed on in part well done prospective randomized trials but patient selection, risk stratification and omega-3-fatty acid dosages have probably a high impact on the different results. However, the concept of omega-3-fatty acid supplementation starts 50 years ago with the observation of two Danish researchers of the low cardiovascular risk of Greenlandic West-coast Eskimos [11]. We have to realize that Eskimos have high omega-3-fatty acid intake in their whole life beginning in utero and during breastfeeding and not only after the first myocardial infarction like in many current studies. A recent published study shows that the concentration of Omega-3-fatty acids in human breast milk is related to their habitual but not current intake [12] and depends on maternal pre-pregnancy body mass index [13]. However, maternal omega-3-fatty acid supplementation improve the DHA and EPA levels in their milk [14]. Pregnant women in Germany had a mean Omega-3 Index below the target range suggested for cardiovascular disease of 8-11%, with large interindividual variation [15,16]. All this is well known and omega-3-fatty acid supplementation in pregnancy have a proven benefit for example to improve neurodevelopmental outcome and allergy prevention but it is not generally recommended and covered by the health insurances. Introduction of complementary food usually leads to decreasing intakes of longchain n-3 polyunsaturated fatty acids, compared to full breastfeeding [17] and may probably explain the beginning of the excessive weight gain in our case report with an age of 6 month. There is evidence of wide variation in per capita dietary intake for both DHA food sources, with low intakes of meat and seafood products being highly prevalent in most low-income countries.
As shown in case 2, omega-3-fatty acid supplementation seems to be beneficial in a 3 years old boy with severe early obesity due to monogenetic obesity of cause a leptin receptor deficiency. Payahoo L et al. proof the effect of omega-3-polyunsaturated fatty acid supplementation on serum leptin levels, appetite sensations, and intake of energy and macronutrients in obese adults in a randomized clinical trial [18]. The mean caloric and macronutrient intakes were decreased significantly in the intervention group but BMI decreased and serum leptin levels increased non significantly.
Robertson RC et al. used a transgenic mouse model and found that maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota [19]. In accordance, Omega-3 fatty acids prevent early-life antibiotic exposureinduced gut microbiota dysbiosis and later-life obesity [20].
With respect to the growing number of obese children and the impact of obesity on mortality in the COVID-19 pandemic, the possibility to improve this urgent problem with a low cost and low risk intervention with omega-3-fatty acid supplementation in children with early onset obesity should be proofed in more children.
References
- Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. The New England journal of medicine. 2018; 379(14): 1303-12. doi: 10.1056/NEJMoa1803527 [published Online First: 2018/10/04].
- Oshakbayev K, Zhankalova Z, Gazaliyeva M, et al. Association between COVID-19 morbidity, mortality, and gross domestic product, overweight/ obesity, non-communicable diseases, vaccination rate: A cross-sectional study. Journal of infection and public health. 2022; 15(2): 255-60. doi: 10.1016/j.jiph.2022.01.009 [published Online First: 2022/01/23].
- Vogel M, Geserick M, Gausche R, et al. Age- and weight groupspecific weight gain patterns in children and adolescents during the 15 years before and during the COVID-19 pandemic. International journal of obesity (2005). 2022; 46(1): 144-52. doi: 10.1038/s41366-021-00968-2
- Lindberg L, Danielsson P, Persson M, et al. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS medicine. 2020; 17(3): 1003078. doi: 10.1371/journal.pmed.1003078 [published Online First: 2020/03/19].
- Lindgren M, Robertson J, Adiels M, et al. Resting heart rate in late adolescence and long term risk of cardiovascular disease in Swedish men. International journal of cardiology. 2018; 259: 109-15. doi: 10.1016/j.ijcard.2018.01.110 [published Online First: 2018/03/27].
- 6. Buchhorn R, Baumann C, Gündogdu S, et al. Diagnosis and management of an inappropriate sinus tachycardia in adolescence based upon a Holter ECG: A retrospective analysis of 479 patients. PloS one. 2020; 15(8): 0238139. doi: 10.1371/journal.pone.0238139 [published Online First: 2020/08/28].
- Zhang W. Chronotropic effects and mechanisms of long-chain omega-3 polyunsaturated fatty acids on heartbeat: the latest insights. Nutrition reviews. 2021; 80(1): 128-35. doi: 10.1093/nutrit/nuab009 [published Online First: 2021/04/11].
- Buchhorn R. Assessment of Biological Effectiveness of Omega3-Fatty Supplements Using Holter ECG Analysis in Children. Ann Nutr Disord & Ther. 2020; 7(1): 1063.
- Baumann C, Rakowski U, Buchhorn R. Omega-3 Fatty Acid Supplementation Improves Heart Rate Variability in Obese Children. International journal of pediatrics. 2018; 2018: 8789604. doi: 10.1155/2018/8789604 [published Online First: 2018/04/24].
- Kohlsdorf K, Nunziata A, Funcke JB, et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. International journal of obesity (2005) . 2018; 42(9): 1602-09. doi: 10.1038/s41366-018-0049-6 [published Online First: 20180227].
- Dyerberg J, Bang HO, Nielsen JA. Plasma lipids and lipoproteins in patients with myocardial infarction and in a control material. Acta medica Scandinavica. 1970; 187(5): 353-63. doi: 10.1111/j.0954-6820.1970.tb02956.x [published Online First: 1970/05/01].
- Bzikowska-Jura A, Czerwonogrodzka-Senczyna A, JasińskaMelon E, et al. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients. 2019; 11(7): 10.3390/nu11071585 [published Online First: 2019/07/25].
- Gustafsson HC, Holton KF, Anderson AN, et al. Increased Maternal Prenatal Adiposity, Inflammation, and Lower Omega-3 Fatty Acid Levels Influence Child Negative Affect. Frontiers in neuroscience. 2019; 13: 1035. doi: 10.3389/fnins.2019.01035 [published Online First: 2019/10/22].
- Amaral YN, Marano D, Silva LM, et al. Are There Changes in the Fatty Acid Profile of Breast Milk with Supplementation of Omega-3 Sources? A Systematic Review. Revista brasileira de ginecologia e obstetricia : revista da Federacao Brasileira das Sociedades de Ginecologia e Obstetricia. 2017; 39(3): 128-41. doi: 10.1055/s-0037-1599094 [published Online First: 2017/03/21].
- von Schacky C. Omega-3 Fatty Acids in Pregnancy-The Case for a Target Omega-3 Index. Nutrients. 2020; 12(4): 10. 3390/nu12040898 [published Online First: 2020/04/01].
- Hoge A, Bernardy F, Donneau AF, et al. Low omega-3 index values and monounsaturated fatty acid levels in early pregnancy: an analysis of maternal erythrocytes fatty acids. Lipids Health Dis. 2018; 17(1): 63. doi: 10.1186/s12944-018-0716-6 [published Online First: 2018/04/03].
- Forsyth S, Gautier S, Salem N, Jr. Dietary Intakes of Arachidonic Acid and Docosahexaenoic Acid in Early Life - With a Special Focus on Complementary Feeding in Developing Countries. Annals of nutrition & metabolism. 2017; 70(3): 217-27. doi: 10.1159/000463396 [published Online First: 2017/03/17].
- Payahoo L, Ostadrahimi A, Farrin N, et al. Effects of n-3 Polyunsaturated Fatty Acid Supplementation on Serum Leptin Levels, Appetite Sensations, and Intake of Energy and Macronutrients in Obese People: A Randomized Clinical Trial. J Diet Suppl. 2018; 15(5): 596-605. doi: 10.1080/19390211.2017.1360975 [published Online First: 20171005].
- Robertson RC, Kaliannan K, Strain CR, et al. Maternal omega-3 fatty acids regulate offspring obesity through persistent modulation of gut microbiota. Microbiome. 2018; 6(1): 95. doi: 10.1186/s40168-018-0476-6 [published Online First: 2018/05/26].
- Kaliannan K, Wang B, Li XY, et al. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. International journal of obesity. (2005),2016; 40(6): 1039-42. doi: 10.1038/ijo.2016.27 [published Online First: 2016/02/16].