Review ArticleOpen Access, Volume 2 Issue 1
Potential of Exosomes as Therapeutics and Therapy Targets in Cancer Patients
Heidi Schwarzenbach*
University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
*Corresponding author: Heidi Schwarzenbach
University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Received : Nov 21, 2023 Accepted : Jan 04, 2024 Published : Jan 11, 2024
Epidemiology & Public Health - www.jpublichealth.org
Copyright: Schwarzenbach H © All rights are reserved
Citation: Schwarzenbach H. Potential of exosomes as therapeutics and therapy targets in cancer patients. Epidemiol Public Health. 2024; 2(1): 1026.
Abstract
After an initial positive response to chemotherapy, cancer patients often acquire chemoresistance and tumor relapse which make cancer to the most lethal diseases worldwide. Exosomes are essential mediators of cell-to-cell communication by delivering their cargo, such as proteins, RNAs and DNA from cell to cell. They participate in cancer progression, metastasis, immune response and therapy resistance. Their ability to shuttle between cells make them to efficient drug delivery systems. As drug transporters, they provide novel strategies for cancer therapy by advancing targeted drug therapy and improving the therapeutic effects of anti-cancer medications. With this review, a comprehensive overview on the potential of exosomes as therapeutics agents and targeted molecules in the treatment of cancer patients is given. The current challenges of preparation of loading exosomes with drugs and delivering them to the recipient tumor cells as well as a consequent exosome-mediated cancer therapy are also discussed.
Introduction
Cancer progression is a multi-step process and eventually leads to the development of metastases and patient death. In 1863, Virchow hypothesized that cancer originates at sites of chronic inflammation, because these regions were observed to cause enhanced cell proliferation [1]. Nowadays, it is recognized that proliferation of cells alone does not cause cancer. Continued cell proliferation occurs in an environment rich of growth/ survival factors, activated stroma and DNA-damage-promoting agents as well as inflammatory cells which may cause neoplastic risk. As soon as a cluster of neoplastic cells is established, angiogenesis, the development of blood vessels, occurs to provide the tumor with oxygen, nutrients and growth factors, as well as to allow to disseminate to distant organs [2]. The invasion and metastasis of solid tumors is accompanied by the epithelial-mesenchymal transition (EMT) [3], a process in which epithelial cells acquire mesenchymal features for their movement to distant organs. Metastatic dissemination can occur early in the malignant progression. In the last decade, the 5-year survival of tumor patients has increased since solid tumors are earlier detected, locally confined and treated by improved adjuvant therapies. However, the clinicians are confronted with an increase in late relapse rates, prolonged disease courses and chemoresistance. Therefore, new targeted therapies have to be developed. Such therapies could be performed by therapeutic exosomes loaded with tumor-specific drugs or genetic material, that in turn are specifically directed to the tumor [4]. Exosomes are a part of the tumor microenvironment and participate in this process regulating multiple tumor stages, for example angiogenesis, immune response, chemoresistance epithelial–mesenchymal transition (EMT) and metastasis. To date, tumor cells release higher levels of exosomes than normal healthy cells [5].
In 1983, exosomes were first detected in maturing mammalian reticulocytes by Harding et al. [6] and Johnstone et al [7]. Later, in 1987, the name of ‘exosomes’ was given by Johnstone et al. [7]. During the maturation process of reticulocyte into erythrocytes, exosomes were observed to selectively remove plasma membrane proteins. In this respect, their lipid composition contains high sphingomyelin content characteristics [8].
Exosomes are a subgroup of extracellular vesicles (EVs). They are small at size of 30-200 nm and of endosomal origin. They mediate intercellular communication by transferring their cargo containing proteins, RNA, DNA and lipids from cell to cell. By delivering these molecules to neighboring and distant cells, they may influence the phenotype of recipient cells. Since their content present the features of the cell of origin, they may transmit the characteristics of the cell of origin. Thus, the movement of exosomes from cancer cells may propagate cancerous attributes to healthy cells and can help cancer cells to spread genetic information leading to the development and maintenance of metastases [9]. Exosomes can be found in different body fluids, including a blood, bronchoalveolar lavage, breast milk, malignant effusions, urine and nasal lavage and formed among others by lymph cells, blood platelets, mast cells, dendritic cells, nerve cells, astrocytes and tumor cells, indicating they can shuttle in different body regions [10].
So far, a variety of methods has been used for the extraction and purification of exosomes by different studies. Techniques for the preparation of exosomes include differential, density and gradient centrifugation, size exclusion chromatography, filtration, polymer-based precipitation and isolation by filtering and chips and have been described in detail by Shtam et al. [11]. In addition, immunological separation using a wide range of antibodies, such as the exosomal surface markers tetraspanins (CD9, CD63, CD81, CD82) and heat-shock proteins (Hsp60, Hsp70, and Hsp90), as target molecules with both magnetic beads and nanowires are also reliable methods [12]. Numerous laboratories use commercial kits based on polymers which can be carried out in a few steps. The main advantage of these kits is a quick purification and the high yields of the exosomes, but their disadvantage is the co-precipitation of proteins of nonexosomal origin. However, the gold standard method seems to be the ultracentrifugation method of isolation, but this technique is labor-intensive and time-consuming and leads to lower exosome amounts. Quality, concentration and biological activity of the extracted exosomes can be verified by Western blot, Nanoparticle Tracking Analysis (NTA), and confocal microscopy [13-15].
Considering the involvement of exosomes in cancer development and progression, the translation of exosome shuttle into the cancer therapy may be of clinical relevance. Since exosomes can deliver their cargo to specific cells, investigation of these vehicles for targeted drug or signal delivery may be a promising approach. Thus, exosomes as carriers of anti-tumor compounds may be promising in the treatment of tumors. In addition, an alternative therapy approach is to target cancer-derived exosomes to prevent cancer progression [16,17].
In this review, a comprehensive overview on the pivotal roles of exosomes in their potential clinical application as novel therapeutic agents and targets is presented. Improved treatment strategies to enhance drug effects mediated by improved drug delivery of this vehicles to the region of the disease are also described.
Exosome biogenesis
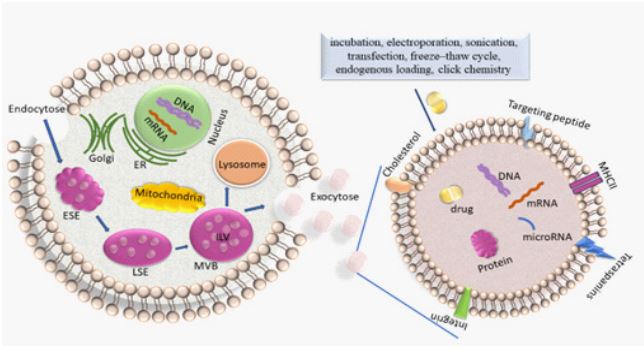
Exosomes are created from the endosomal pathway (Figure 1). Beginning with the (receptor-mediated) endocytosis of extracellular materials by the invagination of the plasma membrane, vesicles are formed and converged into early endosomes. These early endosomes contain extracellular components and plasma membrane proteins. Their maturation into late endosomes occurs by ATPase-mediated acidification. Late endosomes can even obtain cargo-loaded vesicles from the Trans-Golgi network. Multivesicular bodies (MVBs) mature from late endosomes through membrane in-folding processes to form small intra-vesicular vesicles (IVLs). To date, diverse mechanisms of generation of IVLs have been described. For example, MVB formation is coordinated by ESCRT (endosomal sorting complex required for transport) which encompasses four soluble multi-protein complexes, namely ESCRT-0, ESCRTI, ESCRT-II and ESCRT-III [18]. It is initiated by the binding of ubiquitinated proteins to the ESCRT-0 subunit in the endosomal membrane. The association with the cytosolic side of the endosomal membrane allows that particular proteins are sorted into ILVs. Alternatively, another course of EV formation occurs in the absence of ESCRT and is based on ceramide- or tetraspaninenriched microdomains within the endosomal membrane [19]. Upon fusion of the MVB with the plasma membrane, the ILVs which are contained within the MVBs are released into the extracellular space as exosomes. The subsequent DNA and RNA uptake in the recipient cell occurs by either membrane fusion followed by release of these molecules in the cytoplasm or by macro-pinocytosis or receptor/raft-mediated endocytosis. This uptake of exosomes by neighboring and distant cells occurs by docking of the exosomes with specific proteins, sugars, and lipids or by micro-pinocytosis. In turn, the internalized exosomes are targeted to the endosomes which release their content in the recipient cell. The cargo containing genetic material and proteins is then active in guest cell.
Alternatively, MVBs can also fuse with lysosomes leading to the degradation of their contents [5] (Figure 1).
Characteristics of exosomes and their cargo
The exosome membrane resembles the origin cells from which the exosomes are released. The phospholipid belayer consists of diverse proteins, such as anexin II, heat shock proteins, major histocompatibility complex (MHC) class II complexes, integrins and tetraspanins [20]. Since the cell-specific proteins are dependent on the origin cell, for example, exosomes derived from B-lymphocytes are also enriched in MHC-II peptides [21], while exosomes derived from glioma cells carry epidermal growth factor receptor (EGFR) [22].
In the blood circulation, exosomes are stable because of their negatively charged phospholipid membrane and their ability to circumvent the mononuclear phagocytic system by presenting the surface marker CD47 [23]. Amazingly, exosomes have the aptitude even to cross the brain barrier [24,25].
Exosomes contain different components, such as RNAs, including among others mRNAs, microRNAs (miRNAs) and long noncoding RNAs (lncRNA) (small nuclear RNAs), proteins, ceramides and cholesterol, lipids and DNA [17]. Considering the movement of exosomes from tumor cells to healthy cells to propagate genetic information leading to tumor progression, their content of exosomal RNAs and proteins are of particular interest [9]. They may alter the characteristics of the recipient cells to which they are transported [26].
Exosomes contain varying amounts of RNAs concerning over a dozen different RNA forms, miRNAs form the predominant amounts. They belong to the non-coding RNAs (ncRNAs) and are not translated into proteins, but they function in both RNA silencing and post-transcriptional regulation of gene expression [27]. In this respect, they inhibit the expression of mRNA. MiRNA and mRNA are incorporated into an argonaute-containing protein complex termed RISC (RNA-induced silencing complex) in which the mRNA silencing takes place. The RISC was also found in exosomes [28]. MiRNAs can also interact with another ncRNA, namely lncRNAs, and so abrogate the regulatory effects mediated by lncRNAs [29,30].
For example, miR-155 is a highly conserved and an early detected miRNA. It exhibits unique expression profiles and multifunctionality. This miRNA can bind to a repertoire of over 241 genes and so influence diverse signaling pathways. MiR-155 plays a critical role in various physiological and pathological processes, notably in cancer [31].
Besides, studies have shown that specific cell derived exosomes transfer their biological cargo that regulates a variety of processes, and so exosomes are involved in diverse processes, e.g., in tumor progression, angiogenesis, tissue repair and immune functions [32]. These specific characteristics make exosomes to a promising source of a cellular therapy for various conditions, which can be taken advantage by further engineering these exosomes for the delivery of therapeutics. Hereby, it is important to mention that the origin cell which produces exosomes can be modulated by therapeutics as well as by targeting ligands to be loaded.
Loading of exosomes with drug or nucleic acids
Studies have shown that the contents of exosomes can lead to tumor progression, invasion and metastasis [9,33]. By specifically manipulating their cargo, exosomes can be excellent carriers of therapeutics because they are body-intrinsic molecules and so may have potential biocompatibitly with the different body regions. Besides, they possess further advantages over traditional synthetic delivery vectors, including better stronger stability, lower immunogenicity and high stability in the blood circulation. Moreover, loading genetic material into exosomes stimulates the immune system [16]. They can directly deliver drugs to cells because their lipid bilayer structure can be modified to enhance their targeting specificity [34].
There are different strategies for exosome drug loading, including incubation, electroporation, transfection, sonication, freeze–thaw cycle, endogenous loading and click chemistry (Figure 1) [35].
For example, the incubation and electroporation method are carried out by the co-incubation of exosomes with small polar molecules. The loading efficiency depends on the polarity of these molecules. Following isolation and purification of exosomes, purified exosomes are mixed with a specific hydrophobic drug or nucleic acids and then incubated or electroporated. The applied electric charge disrupts the exosome membrane and forms transient, temporary micro-pores in the membrane. These micro-pores increase membrane permeability and develop an electrical potential, which facilitates efficient loading of the drugs. However, the major disadvantages of electroporation are the apoptotic cells caused by the high voltage applied. To confirm the encapsulation efficiency, the absorption intensity of free drugs, exosomes and drug-loaded exosomes are analyzed by a UV-spectrophotometer. In addition, the fluorescence spectra can also be carried out to confirm the successful loading of drugs. Afterwards, the co-localization of drugs and fluorescence-labeled exosomes can be observed in cell cultures by fluorescence microscopy. Liang et al. loaded exosomes with 5-Fluorouracil (5-FU) and the miRNA inhibitor for miR-21 by electroporation. When administered to mice bearing 5-FU resistant colon tumors, the exosomes enhanced cytotoxicity and antitumor activity [36].
The transfection method involves the transfection of molecules, especially small proteins, small interfering RNAs (siRNAs) or miRNAs into the parent cells and the cell packaging into exosomes. Kooijmans et al. transfected neuroblastoma cells with human glycosylphosphatidylinositol (GPI)-anchored protein decay-accelerating factor fused to targeting ligands for epidermal growth factor receptor (EGFR), and showed that the cells produced exosomes displaying EGFR-targeting ligands on their surface [37].
Another method for exosome loading is sonication which uses high-frequency ultrasound energy to partly disrupt the exosome membrane, helping diffusion of therapeutic agents. Kimet al. sonicated a mixture of exosomes and paclitaxel with a 20% amplitude, 6 cycles of 30 s on/off for three minutes with a twominute cooling period between each cycle. They showed that a high amount of paclitaxel could be loaded into exosomes which was measured by a high-performance liquid chromatography. In a model of murine Lewis lung carcinoma pulmonary metastases, the exosomes successfully integrated with paclitaxel by sonication demonstrated a nearly complete co-localization of delivered exosomes with the cancer cells and a potent anticancer effect [38].
Freeze-thaw involves the incubation of exosomes and drugs at room temperature and below freezing temperatures (e.g., -80°C) for several cycles. The freeze-thaw cycles permeabilize the membrane and allow the drugs to enter the exosome membrane. Sato et al. [39] used the freeze-thaw method to fuse exosomes and liposomes containing connexin. Fusion of the exosomes with liposomes to form exosome-liposome hybrids could significantly increase their loading capacity and the half-life of exosomes in plasma.
The endogenous loading method uses small drug molecules that are co-incubated with donor cells or treated with other loading strategies so that they can be absorbed by the cells through the lipid bilayer and encapsulated in exosomes. The resulting exosomes contain the desired small-molecule drugs [35].
Using the click chemistry, small molecules and macromolecules can be attached to the exosome surface by covalent chemical bonds. An example of such a bioconjugation is copper-catalyzed azide-alkyne cycloaddition which is a relatively rapid technique specific for the action between an alkyne and an azide to form a triazole linkage [40].
Delivery of drug-loaded exosomes to cancer cells
As soon as the exosomes are loaded with the particular therapeutic, they have to be directed to the target cell. However, the targeted delivery of drugs to recipient cancer cells is one of the major challenges in cancer therapy research. Typically, nanoparticles as drug carriers are used. They possess prolonged bioavailability, an enhanced permeation and retention effects on tumors, as well as little side effects. Siemer et al. [41] identified the ion channel LRRC8A as a critical component for cisplatin resistance of head and neck cancer patients. To overcome LRRC8A-mediated cisplatin resistance, they constructed cisplatin-loaded, polysarcosine-based core cross-linked polymeric nanoparticles with low immunogenicity, low toxicity and prolonged in vivo circulation. By circumventing the LRRC8A-transport pathway via the endocytic delivery route, the directed delivery of cisplatin by these nanoparticles was able to overcome cisplatin resistance and successfully eliminated cancer cells in a cell spheroid model of head and neck cancer. Lu et al. [42] observed that copper (II) bis(diethyldithiocarbamate) nanoparticles successfully induced copper-dependent programmed cell death in non-small lung cancer cells and potent anti-tumor effects in a cisplatin-resistant tumor model in vivo.
As demonstrated by Ye et al. [43], exosomes combined with low-density lipoprotein (LDL) could ameliorate their uptake by a human primary glioma cell line and permeation into threedimensional glioma spheroids in contrast to unmodulated exosomes. In vivo imaging experiments revealed that LDL could obviously promote exosome extravasation across the blood brain barrier and distribution at the glioma site. The conjugation of apolipoprotein A-1 peptides with lipids allowed for the targeted delivery of methotrexate-loaded exosomes to primary glioma cells.
Triple-negative breast cancer (TNBC) is the most metastatic and recurrent subtype of breast cancer. Owing to the lack of estrogen and progesterone receptors as well as human epidermal grow factor receptor 2 (HER2) and consequently, to the lack of therapeutic targets, chemotherapy and surgical intervention are the only treatments for TNBC. Li et al. [44] constructed a macrophage-derived exosome-coated poly (lactic-co-glycolic acid) nanoplatform that successfully targeted TNBC cells that overexpressed the mesenchymal-epithelial transition factor (c-Met) in vitro and in vivo. The engineered exosome-coated nanoparticles significantly improved the cellular uptake efficiency and the antitumor efficacy of doxorubicin.
Since the uptake of exosomes by recipient cells occurs among others by receptor-mediated binding, their surface can be modulated for cell specific targeting via ligand-receptor binding. For pancreatic cancer therapy, Faruque et al. [45] engineered human pancreatic cancer cell-derived exosomes by conjugating the functional ligand Arg-Gly-Asp (RGD) and magnetic nanoparticles onto their surface. The enhanced therapeutic effect was attributed to the modulation of the exosome surface using RGD, which has an affinity for the highly expressed αvβ3 integrin in pancreatic cancer cells. The RGD-modified autologous exosomes effectively penetrated and internalized tumor cells, and eventually regressed the tumors, by mediating spontaneous removal of α-smooth muscle actin and collagen type 1 in the extracellular matrix of mouse xenografts.
For the specific drug delivery to lung tumors, Pham et al. [46] coupled exosomes with EGFR-targeting peptides and nanoantibodies via protein ligases which facilitated the specific uptake by EGFR-positive lung cancer cells. Systemic delivery of paclitaxel by EGFR-targeting exosomes at a low dose significantly increased drug efficacy in a xenografted mouse model of EGFRpositive lung cancer.
Zhou et al. [47] used exosomes-based biomimetic nanoparticle and designed hybrid exosomes loaded with the protein kinase inhibitor dasatinib by fusing human pancreatic cancer cells derived exosomes with dasatinib-loaded liposomes. Dasatinibloaded hybrid exosomes exhibited significantly higher uptake rates and cytotoxicity to parent pancreatic ductal adenocarcinoma cells than free drugs or liposomal formulations.
A further option for exosome delivery is the use of antibody like affibodies which are based on the immunoglobulin binding domain of protein A. Exosomes that express HER2-affibodies from genetically engineered human embryonic kidney donor cells on their surface successfully delivered paclitaxel and miR21 to HER2-expressing colorectal cancer cells and showed antitumor effects in mice (36). Genetically engineered exosomes that express a fragment of interleukin-3 (IL-3) on their surface effectively bound to IL3-receptor-overexpressing chronic myeloid leukemia cells and inhibited cell growth by delivering the tyrosine kinase inhibitor imatinib to the cells [48]. For immunotherapy of hepatocellular cancer, exosome vaccines can be constructed by anchoring hepatocellular cancer-targeting peptides, antigens or immune adjuvants on the surface of exosomes, inducing effective tumor-specific immune responses [49].
In summary, diverse technical platforms have been developed to modulate the exosome membrane by specific molecules for the production of specific exosome delivery systems in different cancer types
Exosomes as therapeutic agents
Since exosomes have unique characteristics, such as stability, low immunogenicity and high biocompatibility, they are ideal candidates in the treatment of cancer patients. In particular, their eligibility in regenerative outcomes of injury and disease treatment have been shown by mesemchymal stem cell-derived exosomes which play an essential role in wound repair, tissue regeneration and immune response by activating several signaling pathways [16,50].
Over the past decades, a variety of synthetic drug delivery systems has been developed and introduced to the market. However, the application of such systems is restricted since they are often inefficient, cytotoxic and/or immunogenic. Since exosomes are natural-occurring vesicles, their unique features can be used as therapeutic agents to induce immunogenic cell death. The probability of using exosomes derived from dendritic cells as a cancer therapeutic vaccine has been tested in two Phase I clinical studies in melanoma and lung cancer patients. These studies demonstrated that exosomes derived from cancer patient dendritic cells, important in the induction of antitumor immunity, can stimulate both T cells and natural killer cells leading to adaptive, innate cellular immune responses, respectively. The relevance of these exosomes is to transfer antigenloaded MHC I and II molecules, and other associated molecules, to naive dendritic cells, to amplify a cellular immune response [51]. They are able to prime antigen-specific CD4 and CD8 Tcells through MHC-I/ and II expression and antigen presentation [52]. In breast cancer, synthetic multivalent antibody retargeted exosomes activate and redirect T-cells to cancer cells that express EGF receptor or HER-2 on their surface by presenting the respective antibodies [53,54].
In this respect, Zhou et al. constructed a delivery system from bone marrow mesenchymal stem cell exosomes. They loaded galectin-9 siRNA into exosomes by electroporation and superficially modified them with oxaliplatin prodrug as an immunogenic cell death trigger. The approach achieved therapeutic efficacy in pancreatic cancer treatment by eliciting anti-tumor immunity through tumor-suppressive macrophage polarization and cytotoxic T lymphocytes recruitment and downregulating regulatory T cells (Tregs), a T-cell population that suppresses the immune response and maintains immune homeostasis [55].
CD40 signaling is critical in the activation of dendritic cells. Wang et al. [56] identified exosomes from CD40 ligand gene‑modified lung tumor cells to be more immunogenic than unmodified exosomes. These modified exosomes induced a more mature phenotype of dendritic cells and promoted them to secrete high levels of IL‑12. In a mouse model, they induced robust tumor antigen‑specific CD4+ T cell proliferation and enhanced the anti-tumor activity of T-cells.
For individualized immunotherapies, Li et al. [57] developed a nanovaccine platform containing dendritic cell-derived exosomes loaded with patient-specific neoantigens. The nanovaccine elicited potent antigen specific broad-spectrum T-cell and B-cell-mediated immune responses. In particular, the delivery of neoantigen-exosome nanovaccine significantly inhibited tumor growth, prolonged survival, delayed tumor occurrences with long-term memory and eliminated the lung metastasis. Due to presence of exosomal proteins, this exosome-based nanovaccine elicited synergistic antitumor response superior to liposomal formulation.
The therapeutic efficacy of umbilical cord blood-derived M1 macrophage exosomes loaded with cisplatin in ovarian cancer and platinum resistance was investigated by Zhang et al. [58]. In addition, M1 macrophage-derived exosomes carried lnRNA H19, implicated in upregulation of PTEN protein and downregulation of miR-130a and Pgp gene. These engineered exosomes were able to reverse cisplatin drug resistance. Thus, cisplatinloaded M1 macrophage exosomes derived from umbilical cord blood target tumor sites of ovarian cancer can be used to increase the cisplatin sensitivity and cytotoxicity.
In their study, Rehman et al. [59] investigated exosomes isolated from allogeneic bone marrow mesenchymal stem cells treated with heme oxygenase-1 specific short peptide and siRNA nanocarrier for glioblastoma resistant against the cytostatic drug temozolomide. This laboratory showed the excellent tumor cell targeting capability, based on the overexpression of heme oxygenase-1 in glioblastoma, by the modification of bone marrow mesenchymal stem cell-derived exosomes with a specific short peptide for heme oxygenase-1 and loading temozolomide or siRNA into these exosomes.
5-FU is one of the most widely used effective drugs for the treatment of colorectal cancer. To reduce the systemic side effects of 5-FU and chemoresistance, Pang et al. [60] established colorectal cancer cells which overexpressed miR-323a-3p, a tumor suppressor that targets both EGFR and thymidylate synthase. The miR-323a-3p-loaded exosomes could effectively induce apoptosis in colorectal cancer cells by targeting EGFR and thymidylate synthase, and enhanced the therapeutic effects of 5-FU demonstrating the potency of miRNA-loaded exosomes for advanced colorectal cancer biotherapy.
Exosomes as therapeutic targets
Numerous studies have shown the oncogenic potential of cancer-derived exosomes to promote invasion [61], deliver oncogenic genetic material to normal cells [62], increase drug resistance of cancer cells (63,64), and prime distant organs for metastasis [65-67]. In addition, exosomes released from cancerassociated fibroblasts play a role in supporting chemoresistance e.g., in colorectal and breast cancer cells [63,64]. Since such exosomes play a pivotal role in cancer progression, they are also attractive targets in the treatment of cancer patients.
GW4869 is a noncompetitive neutral sphingomyelinase inhibitor that hydrolyzes sphingomyelins to produce ceramides. In addition, GW4869-induced sphingomyelinase inhibition has been reported to inhibit exosome biogenesis and release. Richards et al. [68] showed that the GW4869-mediated inhibition of exosome release from cancer-associated fibroblasts could decrease the chemoresistance and thus, the survival of pancreatic cancer cells. Furthermore, in a mouse model, the combination treatment of gemcitabine and GW4869 resulted in diminished tumor growth. Moreover, Wang et al. [69] developed an assembly of GW4869 and ferroptosis inducer via amphiphilic hyaluronic acid. Treatment with a CD44-targeting nanounit composed of this assembly induced an anti-tumor immune response to melanoma cells in mice, stimulated cytotoxic T lymphocytes and immunological memory and increased the response to programmed cell death-ligand 1 (PD-L1) checkpoint blockade.
The cytostatic drug gemcitabine is a major drug for the treatment of pancreatic ductal adenocarcinoma. However, changes in the levels of specific exosomal miRNAs play an important role in chemoresistance development. RAB27A is a member RAS oncogene family, belongs to the small GTPase superfamily and is involved in protein transport. Targeting of RAB27A with siRNA inhibits exosome secretion and reduce tumor growth and metastasis in mouse models [70,71]. To overcome miR-155-induced gemcitabine of resistance pancreatic ductal adenocarcinoma, Mikamori et al. [72] transfected pancreatic cancer cells with RAB27B siRNA. The reduced exosome release and thus, decrease in miR-155 levels led to a significant decrease in chemoresistance.
Myeloid-derived suppressor cells are a population of immature myeloid cells with the ability to suppress T cell activation. Chalmin et al. [73] prevented the delivery of exosomes containing heat shock protein HSP72 to myeloid-derived suppressor cells by the blood-pressure-lowering drug dimethyl amiloride which reduces endocytic recycling and in turn exosome release. The administration with amiloride could reduce tumor growth in mice and increase cyclophosphamide-based chemotherapy efficacy.
Not only the delivery of exosomes can be inhibited but also the uptake of exosomes can be reduced. Heparan sulfate proteoglycans (HSPGs) serve as receptors of cancer cell-derived exosomes. Christianson et al. [74] observed that the enzymatic depletion of cell surface HSPG effectively attenuated exosome uptake. In addition, Annexin V can also inhibit exosome uptake as well as binding to and blocking surface phosphatidylserine which is important for membrane adhesion. Lima et al. [75] indicated that a highly metastatic melanoma cell line released large quantities of exosomes containing phosphatidylserine. These tumor-derived exosomes increased TGF-β production by cultured macrophages in vitro and enhanced the metastatic potential of melanoma cells in mice. Both effects could be reversed by annexin V. In addition, the treatment could also reduce the tumor growth rate and metastatic potential of human glioma xenografts in mice. However, the analyses with these agents have to be continued because the disruption of other cellular processes, such signaling pathways cannot be excluded.
Likewise, Zhang et al. [76] found that mesenchymal stromal cell-derived exosomes that carry miR-101 could suppress osteosarcoma cell invasion and metastasis by downregulating B-cell lymphoma 6 expression.
On the left side, the biogenesis of exosomes and on the right side, an enhancement of an exosome loaded with a drug are shown. The biogenesis of exosomes is described in the text and comprises endocytosis, MVB formation and exosome secretion into the extracellular microenvironment. Lading of exosomes, as described in the text, is carried out by diverse methods.
ER: endoplasmatic reticulum; ESE: early sorting endosome; LSE: late sorting endosome; MVB: multivesicular body; ILV: intraluminal vesicles.
Conclusion
In the present review article, a short overview on the potential of exosomes in clinical applications as provider of antitumor agents and the development of exosome-derived therapeutic strategies for overcome chemoresistance and tumor progression was presented.
For clinical application, several challenges have to be overcome. First, which cell type is qualified to be used to for exosome extraction and to load them with drugs. For example, the exosome source could be immune cells because exosomes secreted by antigen-presenting cells can confer therapeutic benefits by attenuating or stimulating the immune response. Exosomes derived from dendritic cells can activate T and B cells and carry MHC and so modulate antigen-specific T cell responses [77,78]. A further challenge is to carry out a large-scale production of exosomes [79]. For example, Lamparski et al. [80] developed a quick method for the production, purification and characterization of exosomes derived from antigen presenting cells by ultrafiltration and ultracentrifugation. However, this technique still requires further testing with different types of cells. Next, effective loading approaches have to be standardized [81]. Finally, studies regarding the potency and toxicology of exosomes are essential for bringing them into the clinic. Thus, a comprehensive evaluation of the optimal dose and drug distribution of exosomes in cancer treatment is urgently mandatory. To date, most studies have focused on exosomes in cell experiments in vitro and less often in mice in vivo. Hence, their efficacy and delivery to the recipient cells should be examined on long-term monitoring platforms and in vivo systems. Therefore, large multicenter and longer-term studies are required to achieve their clinical application.
Higher levels of exosomes are particularly secreted by the tumor cells in their environment than by normal cells [82]. The cancer-derived exosomes may in turn be uptaken by normal cells that then may adopt cancerous characteristics. Therefore, a further challenge is to inhibit the tumor-derived exosome secretion and uptake by recipient cells to restore tumor immunity and to impair tumor progression. These investigations should also be carried out by large multicenter and longer-term studies to establish the efficacity of this approach.
In conclusion, the concept of using exosomes as delivery vehicles or tumor-derived exosome as targets may be attractive and promising treatment strategies for fighting cancer.
References
- Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? 2001; 357.
- Sherwood LM, Parris EE, Folkman J. Tumor Angiogenesis: Therapeutic Implications. New England Journal of Medicine. 1971; 285(21): 1182-6.
- Jonckheere S, Adams J, De Groote D, Campbell K, Berx G, Goossens S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. 2022; 211: 157-82.
- Klein CA. Cancer progression and the invisible phase of metastatic colonization. 2020; 20; 681-94.
- Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol [Internet]. 2002; 2(8): 569-79. Available from: http://www.nature.com/articles/nri855.
- Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983; 113(2): 650-8.
- Johnstone RM, Adam M, Hammonds JR, Turbide C. Vesicle Formation during Reticulocyte Maturation. Journal of Biological Chemistry. 1987; 262(1): 9412-20.
- Van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: A common pathway for a specialized function. Vol. 140, Journal of Biochemistry. 2006; 13-21.
- Schwarzenbach H, Gahan P. MicroRNA Shuttle from Cell-ToCell by Exosomes and Its Impact in Cancer. Noncoding RNA [Internet]. 2019; 5(1): 28. Available from: https://www.mdpi.com/2311-553X/5/1/28.
- Harding C V, Heuser JE, Stahl PD. Exosomes: Looking back three decades and into the future. Journal of Cell Biology [Internet]. 2013; 200(4): 367-71. Available from: https://rupress.org/jcb/article/200/4/367/37222/Exosomes-Looking-back-three-decades-and-into-the.
- Shtam T, Evtushenko V, Samsonov R, Zabrodskaya Y, Kamyshinsky R, Zabegina L, et al. Evaluation of immune and chemical precipitation methods for plasma exosome isolation. Patel GK, editor. PLoS One [Internet]. 2020; 15(11): 0242732. Available from: https://dx.plos.org/10.1371/journal.pone.0242732.
- Lim J, Choi M, Lee H, Kim YH, Han JY, Lee ES, et al. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnology. 2019; 17(1).
- Schwarzenbach H. Methods for quantification and characterization of microRNAs in cell-free plasma/serum, normal exosomes and tumor-derived exosomes. Transl Cancer Res [Internet]. 2018; 7(S2): 253-63. Available from: http://tcr.amegroups.com/article/view/16263/15297.
- Wu Y, Wang Y, Lu Y, Luo X, Huang Y, Xie Y, et al. Microfluidic Technology for the Isolation and Analysis of Exosomes. Micromachines (Basel). 2022; 13(10): 1571.
- Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017; 114(40): 10584-9.
- Schwarzenbach H, Gahan PB. Exosomes in Immune Regulation. Noncoding RNA [Internet]. 2021; 7(1): 4. Available from: https://www.mdpi.com/2311-553X/7/1/4.
- Schwarzenbach H, Gahan PB. Predictive value of exosomes and their cargo in drug response/resistance of breast cancer patients. 2020; 3: 63-83.
- Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nature Reviews Molecular Cell Biology. 2020; 21: 25-42.
- Kenific CM, Zhang H, Lyden D. An exosome pathway without an ESCRT. Cell Res. 2021; 31(2): 105-6.
- Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi S EL, et al. Extracellular vesicles as drug delivery systems: Why and how? Advanced Drug Delivery Reviews. 2020; 1593: 32-43.
- Raposo G, Nijman HW, Stoorvogel W, Leijendekker R, Harding C V, Melief CJM, et al. B lymphocytes secrete antigen-presenting vesicles. Journal of Experimental Medicine. 1996; 183(3): 1161-72.
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008; 10(5): 619-24.
- De Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, et al. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc Chem Res. 2019; 52(7): 1761-70.
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011; 29(4): 341-5.
- Cooper JM, Wiklander PBO, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, et al. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Movement Disorders. 2014; 29(12): 1476-86.
- Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics [Internet]. 2013; 14(1): 319. Available from: https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-14-319.
- Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. 2009; 136: 215-33.
- Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell. 2014; 26(5): 707-21.
- Schwarzenbach H, Gahan PB. Interplay between LncRNAs and microRNAs in Breast Cancer. International Journal of Molecular Sciences. 2023; 24: 8095.
- Müller V, Oliveira‐Ferrer L, Steinbach B, Pantel K, Schwarzenbach H. Interplay of lncRNA H19/miR‐675 and lncRNA NEAT1/miR‐204 in breast cancer. Mol Oncol [Internet]. 2019; 13(5): 1137-49. Available from: https://onlinelibrary.wiley.com/doi/10.1002/1878-0261.12472.
- Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiology Biomarkers and Prevention. 2012; 21: 1236-43.
- Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Vol. 306, American Journal of Physiology - Cell Physiology. 2014; 621-33.
- Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol [Internet]. 2009; 21(4): 575-81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0955067409000775.
- Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. 2022; 16: 17802-46.
- Zhan Q, Yi K, Qi H, Li S, Li X, Wang Q, et al. Engineering blood exosomes for tumor-targeting efficient gene/chemo combination therapy. Theranostics. 2020; 10(17): 7889-905.
- Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020; 18(1): 10.
- Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol Res. 2016; 111: 487-500.
- Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016; 12(3): 655-64.
- Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016; 6: 21933.
- Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. 2017; 38: 754-63.
- Siemer S, Bauer TA, Scholz P, Breder C, Fenaroli F, Harms G, et al. Targeting Cancer Chemotherapy Resistance by Precision Medicine-Driven Nanoparticle-Formulated Cisplatin. ACS Nano. 2021; 15(11): 18541-56.
- Lu Y, Pan Q, Gao W, Pu Y, He B. Reversal of cisplatin chemotherapy resistance by glutathione-resistant copper-based nanomedicine via cuproptosis. J Mater Chem B. 2022; 10(33): 6296-306.
- Ye Z, Zhang T, He W, Jin H, Liu C, Yang Z, et al. MethotrexateLoaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Appl Mater Interfaces. 2018; 10(15): 12341-50.
- Li S, Wu Y, Ding F, Yang J, Li J, Gao X, et al. Engineering macrophage-derived exosomes for targeted chemotherapy of triplenegative breast cancer. Nanoscale. 2020; 12(19): 10854-62.
- Al Faruque H, Choi ES, Kim JH, Kim E. Enhanced effect of autologous EVs delivering paclitaxel in pancreatic cancer. Journal of Controlled Release. 2022; 347: 330-46.
- Pham TC, Jayasinghe MK, Pham TT, Yang Y, Wei L, Usman WM, et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J Extracell Vesicles. 2021; 10(4): 12057.
- Zhou Xiaofei, Zhuang Yuetang, Liu Xiaohong, Gu Yaowen, Wang Junting, Shi Yuchen, et al. Study on tumour cell-derived hybrid exosomes as dasatinib nanocarriers for pancreatic cancer therapy. Artif Cells Nanomed Biotechnol. 2023; 51(1): 532-46.
- Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous Leukemia cell growth. Theranostics. 2017; 7(5): 1333-45.
- Zuo B, Zhang Y, Zhao K, Wu L, Qi H, Yang R, et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J Hematol Oncol. 2022; 15(1): 15-46.
- Huang K, Liu W, Wei W, Zhao Y, Zhuang P, Wang X, et al. Photothermal Hydrogel Encapsulating Intelligently Bacteria-Capturing Bio-MOF for Infectious Wound Healing. ACS Nano. 2022; 16(11): 19491-508.
- Delcayre A, Shu H, Le Pecq JB. Dendritic cell-derived exosomes in cancer immunotherapy: Exploiting nature’s antigen delivery pathway. Vol. 5, Expert Review of Anticancer Therapy. 2005; 537-47.
- Yao Y, Fu C, Zhou L, Mi QS, Jiang A. Dc-derived exosomes for cancer immunotherapy. 2021; 13: 3667.
- Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J Am Chem Soc. 2018; 140(48): 16413-7.
- Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Molecular Therapy. 2020; 28(2): 536-47.
- Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021; 268: 120546.
- Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L genemodified lung tumor cells. Mol Med Rep. 2014; 9(1): 125-31.
- Li J, Li J, Peng Y, Du Y, Yang Z, Qi X. Dendritic cell derived exosomes loaded neoantigens for personalized cancer immunotherapies. Journal of Controlled Release. 2023; 353: 423-33.
- Zhang Xiaohui, Wang Jiapo, Liu Na, Wu Weimin, Li Hong, Lu Wen, et al. Umbilical Cord Blood-Derived M1 Macrophage Exosomes Loaded with Cisplatin Target Ovarian Cancer In Vivo and Reverse Cisplatin Resistance. Mol Pharm. 2023; 20(11): 5440-53.
- Rehman FU, Liu Y, Yang Q, Yang H, Liu R, Zhang D, et al. Heme Oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. Journal of Controlled Release. 2022; 345: 696-708.
- Pang Y, Chen X, Xu B, Zhang Y, Liang S, Hu J, et al. Engineered multitargeting exosomes carrying miR-323a-3p for CRC therapy. Int J Biol Macromol. 2023; 247: 125794.
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012; 151(7): 1542-56.
- Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem Biophys Res Commun. 2014; 451(2): 295-301.
- Hu Y, Yan C, Mu L, Huang K, Li X, Tao D, et al. Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS One. 2015; 10(5): 0125625.
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014; 159(3): 499-513.
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015; 527(7578): 329-35.
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015; 527(7576): 100-4.
- Ray K. Pancreatic cancer: Pancreatic cancer exosomes prime the liver for metastasis. Nature Reviews Gastroenterology and Hepatology. 2015; 12: 371.
- Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017; 36(13): 1770-8.
- Wang G, Xie L, Li B, Sang W, Yan J, Li J, et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat Commun. 2021; 12(1): 5733.
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012; 72(19): 4920-30.
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012; 18(6): 883-91.
- Mikamori M, Yamada D, Eguchi H, Hasegawa S, Kishimoto T, Tomimaru Y, et al. MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci Rep. 2017; 7: 42339.
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, RemyMartin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. Journal of Clinical Investigation. 2010; 120(2): 457-71.
- Christianson HC, Svensson KJ, Van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013; 110(43): 17380-5.
- Lima LG, Chammas R, Monteiro RQ, Moreira MEC, Barcinski MA. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009; 283(2):168-75.
- Zhang K, Dong C, Chen M, Yang T, Wang X, Gao Y, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020; 10(1): 411-25.
- Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells., Clinical Medicine Insights: Pathology. 2016; 2016.
- Quah BJC, O’Neill HC. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis. 2005; 35(2): 94-110.
- Van Der Meel R, Fens MHAM, Vader P, Van Solinge WW, EniolaAdefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. Vol. 195, Journal of Controlled Release. 2014; 72-85.
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002; 270(2): 211-26.
- Xi XM, Chen-Meng, Xia SJ, Lu R. Drug loading techniques for exosome-based drug delivery systems. Vol. 76, Pharmazie. 2021; 61-7.
- Schwarzenbach H. Clinical Relevance of Circulating, Cell-Free and Exosomal microRNAs in Plasma and Serum of Breast Cancer Patients. Oncol Res Treat [Internet]. 2017; 40(7-8): 423-9. Available from: https://www.karger.com/Article/FullText/478019.